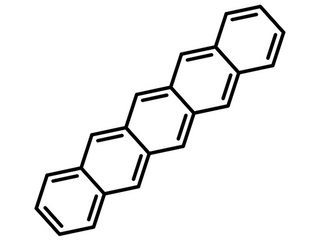
Pentacene
Pentacene, p-type semiconductor in OFETs
Paired with C60 to be used in devices for LED applications, Benzo[b]naphthacene, CAS No. 135-48-8, Sublimed ≥99.0%
Pentacene, an acene with flat-like molecules made of five linearly-fused benzene rings, has been extensively studied as a p-type semiconductor in organic field-effect transistors. It is known to exhibit large carrier mobilities of about 1 cm2 /V s within the plane parallel to the substrate.
Due to its large carrier mobilities, pentacene has also been used with C60 in heterojunction solar cells with a power conversion efficiency over 2.7% [1, 2, 4, 5] and made into devices for light-emitting diode applications [3, 6]
General Information
| CAS number | 135-48-8 |
|---|---|
| Chemical formula | C22H14 |
| Molecular weight | 278.35 g/mol |
| Absorption* | λmax = 576 nm (in benzene) [11] |
| Fluorescence | λem = 578 nm (in benzene) |
| HOMO/LUMO | HOMO -4.9 eV, LUMO -3.0 eV |
| Synonyms |
|
| Classification / Family | Acene derivatives, Hole-injection layer materials, Hole transport layer materials, Phosphorescent host materials, Photovoltaic materials, Sublimed materials, Light-emitting diodes, Light emitting field-effect transistors (LEFETs), OFETs, OPVs, Organic electronics |
* Measurable with an optical spectrometer
Product Details
| Purity | >99% (sublimed) |
|---|---|
| Melting point | 372-374 °C (subl.) |
| Color | purple-black crystals/powder |
| Solvents | Pentacene is insoluble in most of the organic solvents. Trichlorobenzene is normally used to form solution at 60 - 120 °C [10] |
* Sublimation is a technique used to obtain ultra pure-grade chemicals, see sublimed materials.
Chemical Structure
Device Structure(s)
| Device structure | ITO/Pentacene (45 nm)/C60 (50 nm)/BCP (10 nm)/Al [1] |
|---|---|
| Jsc (mA cm-2) | 15±3 |
| Voc (V) | 0.363±0.03 |
| FF (%) | 50±1 |
| PCE (%) | 2.7±0.4 |
| Device structure | ITO/pentacene:CuPc (4:96 wt%, 20 nm)/C60 (60 nm)/BCP (8 nm)/Al (80 nm) [2] |
|---|---|
| Jsc (mA cm-2) | 12.93 |
| Voc (V) | 0.52 |
| FF (%) | 46 |
| PCE (%) | 3.06 |
| Device structure | ITO/PEDOT:PSS/pentacene (10 nm)/Alq3 (30 nm)/Al [3] |
|---|---|
| Color | Green |
| Max. EQE | n/a |
| Max. Current Efficiency | 8.2 cd/A |
MSDS Documentation
Literature and Reviews
- Efficient thin-film organic solar cells based on pentacene/C60 heterojunctions, S. Yoo et al., Appl. Phys. Lett. 85, 5427 (2004); doi: 10.1063/1.1829777.
- Improving efficiency of organic photovoltaic cells with pentacene-doped CuPc layer, W.-B. Chen et al., Appl. Phys. Lett. 91, 191109 (2007); http://dx.doi.org/10.1063/1.2806195.
- Improved performance of organic light emitting diodes by pentacene as hole transporting layer, F. Zhang et al., Appl. Surf. Sci., 255, 1942–1945 (2008), doi:10.1016/j.apsusc.2008.06.166.