9,9-Dioctyl-2,7-dibromofluorene
CAS Number 198964-46-4
Chemistry Building Blocks, Dibromo Monomers, Materials, Monomers, Non-Heterocyclic Building Blocks9,9-Dioctyl-2,7-dibromofluorene, for the synthesis of semiconducting polymers
High quality and high purity monomer available online
Specifications | MSDS | Literature and Reviews
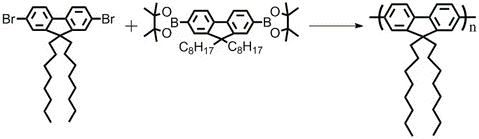
9,9-Dioctyl-2,7-dibromofluorene (CAS number 198964-46-4) can be used for the synthesis of polymer semiconductors, engaging either Suzuki coupling or Stille coupling reactions.
Alkyl-substituted polyfluorenes have emerged as a very attractive class of conjugated polymers, especially for display applications. This is due to their pure blue and efficient electroluminescence, coupled with a high charge-carrier mobility and good processability.
Their unique molecular structure, joined biphenyl at 9-position for a maximum pi-conjugation and planar structure, and also their excellent optical and electronic properties altogether have brought semiconducting polyfluorenes to the focus of scientific and industrial interest. Polyfluorenes are known as semiconducting materials used for light emitting diodes, organic photovoltaics and interface layer materials for organic electronics. By reacting with 9,9-Dioctylfluorene-2,7-diboronic acid bis(pinacol) ester in toluene, it yields PFO (F8).
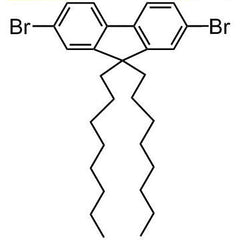
General Information
| CAS Number | 198964-46-4 |
| Chemical Formula | C29H40Br2 |
| Molecular Weight | 548.44 g/mol |
| Synonyms | 2,7-Dibromo-9,9-dioctyl-9H-fluorene 2,7-Dibromo-9,9-dioctylfluorene 2,7-Dibromo-9,9-di-n-octylfluorene |
| Classification / Family | Fluorene, Halogenated fluorene, Organic materials, Semiconductor Synthesis, Low band gap polymers, OFETs, Organic Photovoltaics, Polymer Solar Cells, Polymer light-emitting diodes |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | 59 °C - 63 °C |
| Appearance | Light Brown/White Crystals/Powder |
Characterisation
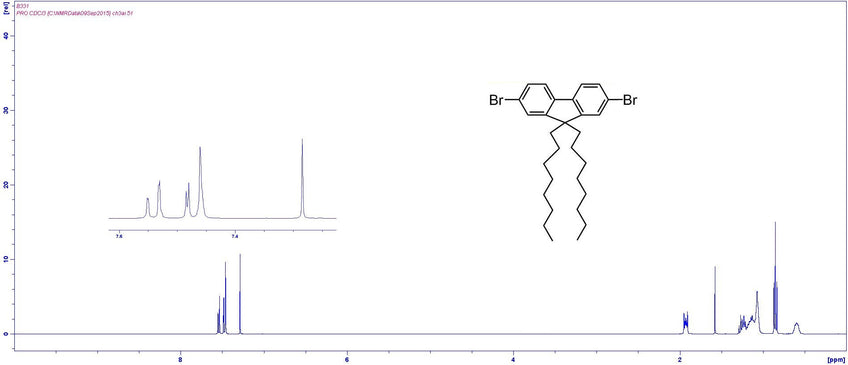
MSDS Documentation
 9,9-Dioctyl-2,7-dibromofluorene MSDS sheet
9,9-Dioctyl-2,7-dibromofluorene MSDS sheet
Literature and Reviews
- Synthesis and Spectroscopy of Poly(9,9-dioctylfluorene-2,7-diyl-co-2,8-dihexyldibenzothiophene-S,S-dioxide-3,7-diyl)s: Solution-Processable, Deep-Blue Emitters with a High Triplet Energy, K. Kamtekar et al., Macromolecules, 43 (10), 4481–4488 (2010).
- Synthesis and electroluminescence properties of fluorene–anthracene based copolymers for blue and white emitting diodes, H. Song et al., J. Industr. and Eng. Chem., 17, 352–357 (2011).
- Enhanced Efficiency of Polyfluorene Derivatives: Organic–Inorganic Hybrid Polymer Light-Emitting Diodes, J. LEE et al., J. Poly. Sci.: Part A: Poly. Chem., 9, 2943 (2006).
