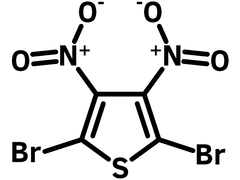
2,5-Dibromo-3,4-dinitrothiophene
CAS Number 52431-30-8
Chemistry Building Blocks, Dibromo Monomers, Heterocyclic Building Blocks, Materials, MonomersA fully functionalised thiophene unit
Used for the synthesis of electron deficient thieno[3,4-b]thiadiazoles or thienopyrazines in application of organic solar cells and photodetectors
Specifications | MSDS | Literature and Reviews
2,5-Dibromo-3,4-dinitrothiophene (CAS number 52431-30-8) is a fully substituted thiophene unit with two electron withdrawing nitro groups at 3,4-positions and two bromo functional groups at 2,5-postions. Nitro groups can be reduced to amino groups to further form thienopyrazines and thieno[3,4-b]thiadiazole. Bromo groups gives the functionality for C-C formation reactions, i.e. Stille or Suzuki couplings.
DPTP-4D, a 2,3-diphenylthieno[3,4-b]pyrazine based small molecule containing tetrakis-triphenyl amino donors, could be synthesised from simple, low cost and readily available 2,5-dibromo-3,4-dinitrothiophene. Being an effective hole-transporting material, DPTP-4D can be used to replace Spiro-OMeTAD and PTAA. It gained a PCE of 20.18% with high environmental, thermal, and light-soaking stability in PSC n-i-p planar devices.
Small molecule photodetectors (SMPDs) based on thieno[3,4-b]thiadiazole, also derived from 2,5-dibromo-3,4-dinitrothiophene showed a high detectivity of 5.0 × 1011 Jones at 800 nm at the bias of -0.1 V. With a sufficiently thin silver electrode, visibly transparent PDs with an average transmittance of 45% in the visible region were fabricated.
Dinitrothiophene building block
for the synthesis of OLED and organic photovoltaic materials
Worldwide shipping
Quick and reliable shipping
Capped with bromides
for facile coupling reactions
High purity
>97% Purity
General Information
| CAS Number | 52431-30-8 |
| Chemical Formula | C4Br2N2O4S |
| Full Name | 2,5-Dibromo-3,4-dinitrothiophene |
| Molecular Weight | 331.93 g/mol |
| Synonyms | 2,5-Dibromo-3,4-dinitro-thiophene |
| Classification / Family | Thiophene derivatives, Semiconductor synthesis intermediates, Low band gap polymers, OLED, OFETs, organic photovoltaics |
Chemical Structure
Product Details
| Purity | >98% (1H NMR) |
| Melting Point | Tm = 137 °C |
| Appearance | White to off-white powder |
MSDS Documentation
 2,5-Dibromo-3,4-dinitrothiophene MSDS Sheet
2,5-Dibromo-3,4-dinitrothiophene MSDS Sheet
Literature and Reviews
-
Synthesis of thienoselenadiazole-containing conjugated copolymers and their application in polymer solar cells, X Shen et al., Polym. J, 44, 978–981 (2012): DOI:10.1038/pj.2012.33.
-
Multicolored Electrochromic Cells Based On Poly(2,7-Carbazole) Derivatives For Adaptive Camouflage, S. Beaupré et al., Chem. Mater., 21, 8, 1504–1513 (2009); DOI: 10.1021/cm802941e.
-
Synthesis and structural characterization of 2,5-dihalo-3,4-dinitrothiophenes, L. Wen et al., J. Chem. Crystallogr., 37, 387–398 (2007); DOI: 10.1007/s10870-006-9160-y.
