Specifications | MSDS | Literature and Reviews | Technical Support
P4T2F-HD belongs to the polythiophenes (PTs) family with either fluoro or alkyl groups on the main backbone of the polymer.
Fluorination can promote stronger interchain interaction to enhance polymer crystallinity. While P3HT can form completely mixed bulk heterjunction (BHJ) films with non-fullerene acceptors, P4T2F-HD has relatively low miscibility with them, i.e. Y6-BO (BTP-4F-12), which enables the blending active materials to form desired packing and nanoscale phase separation morphology for efficient OSCs.
For 5 - 10 grams order quantity please enquire, the lead time is 4-6 weeks.
The Luminosyn™ Range
General Information
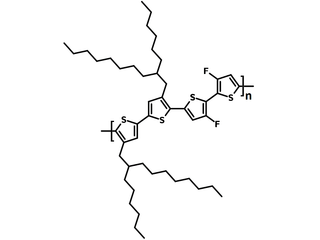
| Full name | Poly(3,3′-dihexyldecyl-4'',4'''-difluoro[2,2′:5′,2′′:5′′,2′′′-quaterthiophene]-5,5′′′-diyl) |
| Synonyms | PQT16-2F, PQTHD-2F, Poly(4,4′-difluoro-3'',3'''-difluoro[2,2′:5′,2′′:5′′,2′′′-quaterthiophene]-5,5′′′-diyl) |
| Chemical formula | (C48H70F2S4)n |
| CAS number | n.a. |
| HOMO / LUMO | HOMO = -5.35 eV, LUMO = -3.43 eV [1] |
| Melting Point | Tm = 182 °C |
| Soluble in | Chloroform, chlorobenzene and dichlorobenzene |
| Recommended Processing Solvents at 10mg/ml | Chlorobenzene |
| Classification / Family | Polythiophenes, Organic semiconducting materials, Medium band-gap polymers, Organic Photovoltaics, OFETs, Polymer solar cells, NF-PSCs. |
Batch Details
| Batch | Mw | Mn | PDI | Stock Info |
| M2348A1 | 44,406 | 29,331 | 1.51 | In stock |
Chemical Structure
Device Structure
Record device performance with PCE of 13.65% was achieved by using an active layer materials with P5TCN-F25 as the polymer donor and Y6-BO as the acceptor by the following device structure with excellent storage and thermal stability.
Device structure: ITO/ZnO/C60-SAM/P4T2F-HD:Y6-BO/MoO3/Ag [1].
| Thickness (nm) | VOC (V) | JSC (mA cm-2) | FF (%) | PCE (%) |
| 100 | 0.72 | 24.39 | 75.30 | 13.34 |
MSDS Documentation
Literature and Reviews
- Surpassing 13% Efficiency for Polythiophene Organic Solar Cells Processed from Nonhalogenated Solvent, J. Xiao et al., 22 (25), 2008158 (2021); Adv. Mater., DOI: 10.1002/adma.202008158.
- Polythiophene derivatives compatible with both fullerene and non-fullerene acceptors for polymer solar cells, X. Jia et al., J. Mater. Chem. C, 7, 314-323 (2019); DOI: 10.1039/C8TC04746D.
- The renaissance of polythiophene organic solar cells, L. Ye et al., Trends Chem., 3 (12), 1074-1087 (2021); DOI: 10.1016/j.trechm.2021.09.008.
