OXD-7
OXD-7, high efficiency ETL material
With PVK, good solubility and film morphology, 1,3-bis[2-(4-tert-butylphenyl)-1,3,4-oxadiazo-5-yl]benzene, CAS No. 138372-67-5, Sublimed ≥99.0%
OXD-7 is a well known electron-transporting material due to the electron-accepting property of the oxadiazole units. Together with poly(9-vinylcarbazole) (PVK, electron donating), OXD-7 (electron withdrawing) is the most widely used hybrid-type host due to its good solubility and film morphology by the bulky tert-butyl units.
OXD-7 has also been used as an ultraviolet emitter, with MoO3 as hole injection and buffer material showing relatively high external quantum efficiency [1].
General Information
| CAS number | 138372-67-5 |
|---|---|
| Chemical formula | C30H30N4O2 |
| Molecular weight | 478.58 g/mol |
| Absorption | λmax 292 (THF) |
| Fluorescence | λem 347 nm (THF) |
| HOMO/LUMO | HOMO = 6.5 eV, LUMO = 3.0 eV |
| Synonyms |
|
| Classification / Family |
Electron-injection materials, Electron transporting materials, Phosphorescent host materials, Organic light-emitting diodes, Organic electronics |
Product Details
| Purity | > 99.0% (sublimed) |
|---|---|
| Melting point | 241 °C (lit.) |
| Color | White powder/crystals |
*Sublimation is a technique used to obtain ultra pure-grade chemicals. For more details about sublimation, please refer to the Sublimed Materials.
Chemical Structure
Device Structure(s)
| Device structure | ITO/PEDOT:PSS/PVK:OXD-7:TPD:(Et-Cvz-PhQ)2Ir(pic)*/OXD-7 (20 nm)/Ba (3 nm)/Al (100 nm) [6] |
|---|---|
| Color | Red |
| Max. Current Efficiency | 17.5 cd/A |
| Max. EQE | 10.6% |
| Max. Power Efficiency | 6.42 lm W−1 |
|
Device structure |
ITO/PEDOT:PSS(40 nm)/mCP:PVK:OXD-7(33:33:22 wt%): (dfpmpy)2Ir(pic-N-O):(F4PPQ)2Ir(pic-N-O): (EO2- Cz-PhQ)2Ir(acac)*(12:0.25:0.15 wt%) (50-60 nm)/TmPyPB(20 nm)/LiF(1 nm)/Al(150 nm) [7] |
| Color | White |
|---|---|
| Max. EQE |
11.45% |
| Max. Current Efficiency | 23.04 cd/A |
| Max. Power Efficiency | 8.04 lm W−1 |
| Device structure | ITO/PEDOT:PSS/NPB/mCP/FPt*(1.5 nm)/OXD-7/CsF/Al [8] |
|---|---|
| Color | White |
| Max. EQE | 17.5% |
| Max. Power Efficiency | 45 lm W−1 |
| Device structure | ITO/ PEDOT:PSS 1.5 (75 nm)/PVK:OXD-7:complex 5 (100:37:8 w/w) (80 nm)/Ba (4 nm)/Al (100 nm) [9] |
|---|---|
| Color | Blue |
| Max. EQE | 8.7% |
| Max. Current Efficiency | 19.1 cd/A |
| Max. Power Efficiency | 6.6 lm W−1 |
| Device structure | ITO/PEDOT:PSS/ PVK :OXD-7:Ir(mppy)3 (60:40:4, w/w)/TrOH*/Al [10] |
|---|---|
| Color | Green |
| Max. Luminance | 18,050 |
| Max. EQE | 6.7% |
| Max. Current Efficiency | 23.4 cd/A |
| Device structure | ITO/MoOx/PVK:OXD-7:FIrpic (70:30:10/B1Mo/Al [11] |
|---|---|
| Color | Blue |
| Max. Luminance | 42,000 |
| Max. EQE | 15.4% |
| Max. Current Efficiency | 30 cd/A |
| Max. Power Efficiency | 12.5 lm W−1 |
| Device structure | ITO/PEDOT:PSS/PVK:OXD-7:FIrpic (60:40:10 w/w, 70 nm)/SPDP* (15 nm) LiF (1 nm)/Al (100 nm) [12] |
|---|---|
| Color | Blue |
| Max. EQE | 19.6% |
| Max. Current Efficiency | 33.6 cd/A |
| Max. Power Efficiency | 10.6 lm W−1 |
*For chemical structure information, please refer to the cited references.
Characterization
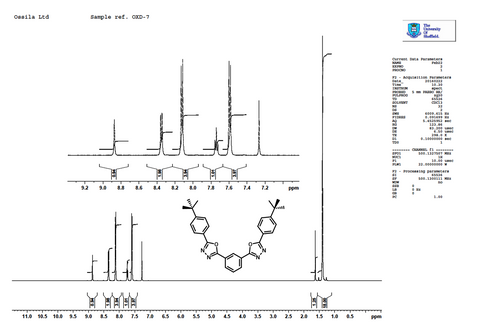
Pricing
| Grade | Order Code | Quantity | Price |
|---|---|---|---|
| Sublimed (>99% purity) | M451 | 250 mg | £180 |
| Sublimed (>99% purity) | M451 | 500 mg | £320 |
| Sublimed (>99% purity) | M451 | 1 g |
£500 |
MSDS Documentation
Literature and Reviews
- <Highly efficient ultraviolet organic light-emitting diodes and interface study using impedance spectroscopy, Q. Zhang et al., Electron Optics, 126 (18), 1595-1597 (2015).
- Small Molecule Host Materials for Solution Processed Phosphorescent Organic Light-Emitting Diodes, K. Yook et al., Adv. Mater., 26, 4218–4233 (2014).
- High power efficiency solution-processed double-layer blue phosphorescent organic light-emitting diode by controlling charge transport at the emissive layer and heterojunction, K. Yeoh et al., Phys. Status Solidi RRL 7, No. 6, 421–424 (2013) / DOI 10.1002/pssr.201307089.

