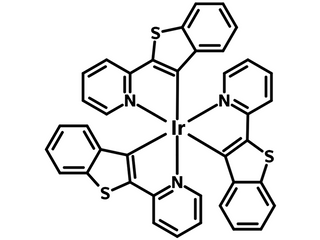
Ir(btpy)3
CAS Number 405289-74-9
Dopant Materials, High Purity Sublimed Materials, Materials, OLED Materials,Ir(btpy)3, efficient red PhOLED
High-purity (>99.0%) and available online for priority dispatch, Tris(2-(benzo[b]thiophen-2-yl)pyridineiridium(III), CAS No. 405289-74-9, Sublimed ≥99.0%
Tris(2-(benzo[b]thiophen-2-yl)pyridineiridium, known as Ir(btpy)3, is an efficient red phosphorescent emitter (PHOLED). It is commonly used in organic light-emitting diodes and polymer solar cells as a dopant, and in barometric pressure probes due to its sensitivity to oxygen [2].
Wang et al. demonstrated that a higher Ir(btpy)3 doping concentration is beneficial to photon harvesting in the active layer, but the interpenetrating network of P3HT:PCBM can be damaged through high-temperature annealing treatment [3].
General Information
| CAS number | 405289-74-9 |
|---|---|
| Chemical formula | C39H24IrN3S3 |
| Molecular weight | 823.08 g/mol |
| Absorption | λmax 292, 366, 408 nm in THF |
| Fluorescence | λem 596, 645 nm in THF |
| HOMO/LUMO | HOMO 5.08 eV; LUMO 2.67 eV [1] |
| Synonyms |
|
| Classification / Family | Organometallic complex, phosphorescent red emitter, phosphorescence dopant OLEDs, polymer solar cells, sublimed materials |
Product Details
| Purity | >99.0% (sublimed) |
|---|---|
| Melting point | > 300 °C |
| Color | Red powder |
*Sublimation is a technique used to obtain ultra pure-grade chemicals. For more details about sublimation, please refer to the Sublimed Materials.
Chemical Structure
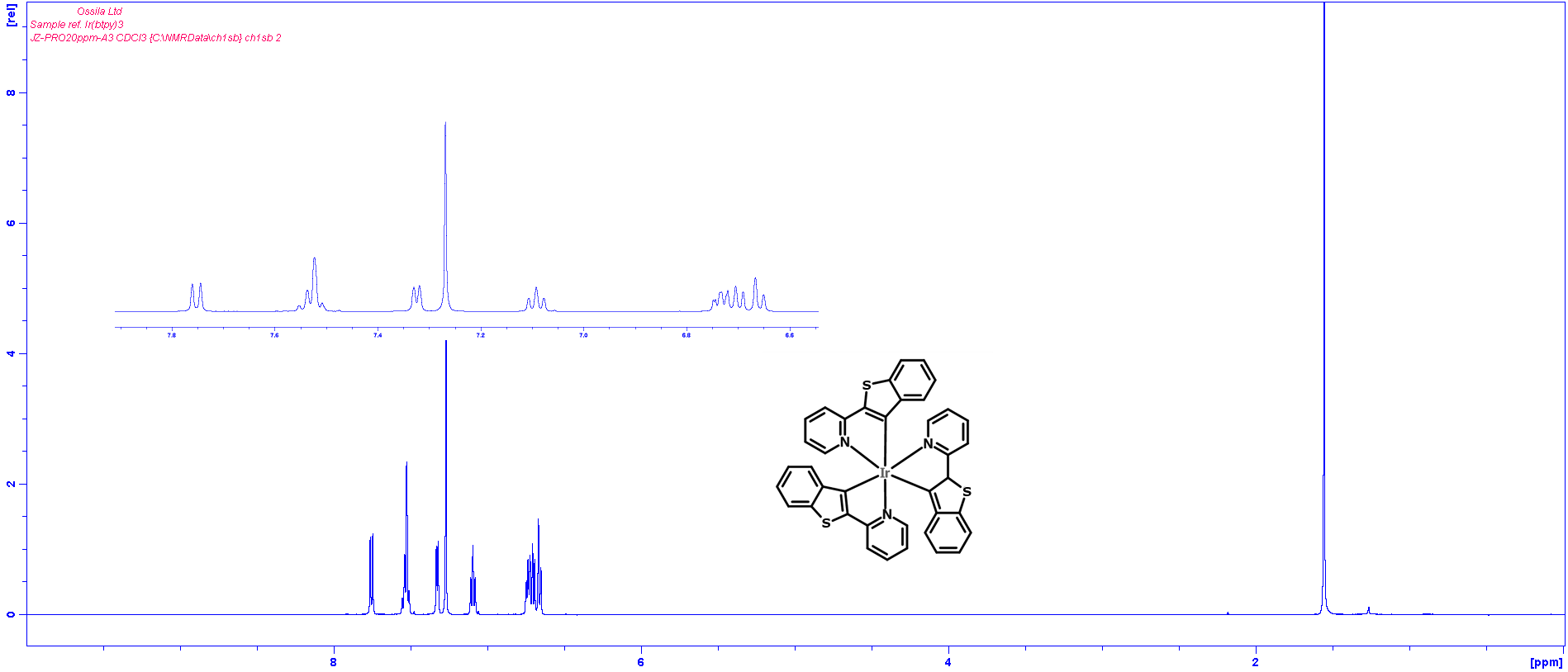
Characterization

Pricing
| Grade | Order Code | Quantity | Price |
|---|---|---|---|
| Sublimed (>99.0% purity) | M491 | 100 mg | £330 |
| Sublimed (>99.0% purity) | M491 | 250 mg | £660 |
| Sublimed (>99.0% purity) | M491 | 500 mg | £1200 |
| Sublimed (>99.0% purity) | M491 | 1 g | £2100 |
MSDS Documentation
Literature and Reviews
- The Red Electroluminescence of Iridium Complex at Different Concentrations and Host Materials, W. Zhang et al., Appl. Mechanics and Mater., Ch. 2, 94-97 (2014); DIO: 10.4028/www.scientific.net/AMM.633-634.94.
- Structure–Property Relationship of Red- and Green-Emitting Iridium(III) Complexes with Respect to Their Temperature and Oxygen Sensitivity, N. Tian et al., Eur. J. Inorg. Chem. 2010, 4875–4885, DOI: 10.1002/ejic.201000610.
- Effect of Doping Phosphorescent Material and Annealing Treatment on the Performance of Polymer Solar Cells, Z. Wang et al., Inter. J. Photoenergy, 273586 (2013), http://dx.doi.org/10.1155/2013/273586.