Low price, high purity IDIC-4F available to buy online
Electron acceptor able to give a device performance efficiency of over 12.1%
Specifications | Pricing and Options | MSDS | Literature and Reviews
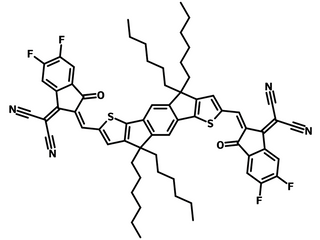
IDIC-4F (CAS number 2229934-71-6), a fluorinated analog of IDIC, shows an A-D-A type structure. The structure has an electron-donating fused five-member-ring indaceno[1,2-b:5,6-b′]dithiophene (IDT) core, terminated by two electron-withdrawing units, 2-(5,6-difluoro-3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile (IC-2F).
All small molecule organic solar cells (SM-OSCs) with H14 as a small molecule donor and IDIC-4F as electron acceptor have an easy to fabricate and simple structure. Such devices have been shown to give a device performance efficiency over 12.1% which is the highest efficiency reported for IDIC-4F-based SM-OSCs.
Device structure: ITO/PEDOT:PSS AI 4083/H14:IDIC-4F/PDINO/Al [2]
| Thickness (nm) | VOC(V) | JSC(mA cm-2) | FF (%) | PCE(%) |
|---|---|---|---|---|
| 85 | 0.943 | 18.3 | 70.2 | 12.1 |
General Information
| CAS Number | 2229934-71-6 |
|---|---|
| Chemical Formula | C66H62F4N4O2S2 |
| Purity | ≥99% (1HNMR) |
| Full Name | 2,2'-((2Z,2'Z)-((4,4,9,9-tetrahexyl-4,9-dihydro-s-indaceno[1,2-b:5,6-b']dithiophene-2,7-diyl)bis(methanylylidene))bis(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile |
| Molecular Weight | 1083.35 g/mol |
| Absorption* | λmax 707 nm (Film) |
| HOMO / LUMO | HOMO = -6.30 eV, LUMO = -3.82 eV [1] |
| Solubility | Chloroform, chlorobenzene |
| Form | Dark purple red powder/crystals |
| Synonyms | ID4F, ID-4F |
| Classification / Family | NFAs, n-type non-fullerene electron acceptors, Organic semiconducting materials, Low band-gap small molecule, Small molecular acceptor, Organic photovoltaics, Polymer solar cells, NF-PSCs |
* Measurable with an optical spectrometer
Chemical Structure
Pricing
| Batch | Quantity | Price |
|---|---|---|
| M2241A1 | 100 mg | £290 |
| M2241A1 | 250 mg | £580 |
| M2241A1 | 500 mg | £990 |
| M2241A1 | 1 g | £1790 |
MSDS Documentation
Literature and Reviews
- Di-fluorinated Oligothiophenes for High-Efficiency All-Small-Molecule Organic Solar Cells: Positional Isomeric Effect of Fluorine Substitution on Performance Variations, T. Duan et al., Solar RRL, 4 (3), 2019; doi: 10.1002/solr.201900472.
- Precise Control of Phase Separation Enables 12% Efficiency in All Small Molecule Solar Cells, H. Bin et al., Adv. Energy Mater., 2001589 (2020); DOI: 10.1002/aenm.202001589.
- As-cast ternary polymer solar cells based on a nonfullerene acceptor and its fluorinated counterpart showing improved efficiency and good thickness tolerance, F. Pan et al., J. Mater. Chem. A, 7, 9798-9806 (2019); doi: 10.1039/C9TA01003C.
Related Products
Semiconducting polymers for bulk heterojunction, OPV, OLED, OFET and perovskite interfaces and solar cell research.


