Guanidinium Bromide
CAS Number 19244-98-5
Materials, Perovskite Materials, Perovskite Precursor MaterialsOverview | Specifications | MSDS | Literature and Reviews | Resources and Support
Guanidinium bromide (GDBr) and its derivatives are known to promote and enable stable 2D perovskite structures. This is done via i) the van der Waals interactions between guanidinium cations, and ii) hydrogen−halogen bonding between guanidinium cations and halide ions of different layers.
The presence of the guanidinium cation results in reduced defect density for the perovskite film and a longer carrier lifetime, due to a hydrogen-bonding effect produced by hydrogen from the guanidinium salt.
General Information
| CAS number | 19244-98-5 |
| Chemical formula | CH6BrN3 |
| Molecular weight | 139.98 g/mol |
| Synonyms | Guanidine hydrobromide, Guanidine monohydrobromide, GDBr |
| Classification / Family | Guanidinium halides, Perovskite precursor materials, Perovskite solar cells, Perovskite LEDs |
Product Details
| Purity | 98% |
| Melting point | 191 °C |
| Color | White Powder/crystals |
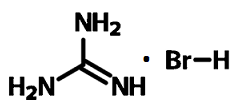
Chemical Structure
MSDS Documentation
Guanidinium bromide MSDS sheet
Literature and Reviews
- Guanidinium: A Route to Enhanced Carrier Lifetime and Open-Circuit Voltage in Hybrid Perovskite Solar Cells, N. De Marco et al., Nano Lett., 16 (2), 1009–1016 (2016); DOI: 10.1021/acs.nanolett.5b04060.
- Guanidinium benefit, O. Graydon, Nat. Photonics 10, 145 (2016); doi:10.1038/nphoton.2016.35.
- Mixed Iodide–Bromide Methylammonium Lead Perovskite-based Diodes for Light Emission and Photovoltaics, L. Gil-Escrig et al., J. Phys. Chem. Lett., 6 (18), 3743–3748 (2015); DOI: 10.1021/acs.jpclett.5b01716.
