DMAcPA
CAS Number 2971088-37-4
Charge Transport Layer Materials, Materials, OLED Materials, Perovskite Interface Materials,Small Self-Assembled Monolayer Molecule for High Efficiency Solar Cells
DMAcPA, Hole transport or extraction layer for NFA-polymer solar cells and p-i-n perovskite solar cells
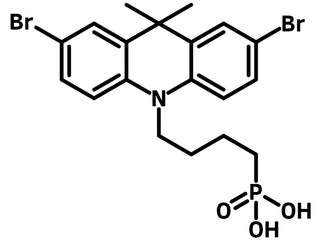
DMAcPA ((4-(2,7-dibromo-9,9-dimethylacridin-10(9H)-yl)butyl)phosphonic acid, CAS No. 2971088-37-4) is a self-assembled monolayer material consisting of a phosphonic acid anchor group, a butyl linker, and a 2,7-dibromo-9,9-dimethyl-9,10-dihydroacridine terminal group. DMAcPA can be engaged as a dopant to perovskite materials or hole transport layer (HTL) by forming self-assembled monolayer (SAM) between the perovskite active layer and the substrate electrode.
Much different to the conventional self-assembled monolayer hole transport layer materials, DMAcPA was created as a p-dopant mixture to the perovskite precursor solutions. The perovskite film doped with DMAcPA shows smoother film morphology and diminished excess PbI2 with reduced deep-level hole trap density by suppressing defect states at grain boundaries and surfaces. The two methyl groups at the acridine terminal unit imposes a steric effect in DMAcPA, preventing aggregation at grain boundaries in the film-formation process. The direct deposition of DMAcPA doped perovskite on ITO substrates enables the formation of strong p-type perovskites, with residual DMAcPA being mainly present at grain boundaries to passivate defect states and the bottom interface to facilitate hole extraction.
DMAcPA simplifies the processing details by doping directly the perovskite precursor solutions without extra layer of PEDOT:PSS, PTAA or Spiro-MeOTAD though DMAcPA can also be processed as a standing alone hole extraction layer. Device efficiency with a PCE of 25.86% (reverse scan) was achieved by doping perovskite active layer (Perovskite+DMAcPA). The fabricated device maintained 96.6% of its initial PCE after 1,000 h of light soaking.
Serving as hole selective contact for organic solar cells and perovskite solar cells, DMAcPA is an alternative to PEDOT:PSS with superior performance with the convenience of solution deposition at low concentration, i.e. 1 mM.
Solution Processing Procedure
DMAcPA as standing alone self-assembly monolayer.
Typical processing solvents: THF, anhydrous ethanol (IPA, IPA/DMF are also superior solvents)
Typical concentration: 1.0 mg/ml (0.5 - 3.0 mg/ml)
Typical processing procedure: DMAcPA is dissolved in THF at a concentration of 1.0 mg/ml. The solution is spin-coated on the substrate for 30 s at 3000 rpm in a nitrogen filled glove box. The DMAcPA coated substrate is then annealed for 10 min at at 100 ℃.
DMAcPA as dopant to the perovskite solution.
Typical processing solvents: DMF and DMSO solution (4:1, v/v)
Typical doping concentration: 4.0 mg/ml (1.0 - 7.0 mg/ml)
Typical processing procedure: Perovskite precursor solution is made up by dissolving the precursors in DMF and DMSO solution (4:1, v/v). The solution is heated and stirred at 60 ℃ for 1 h to prepare undoped precursor solution. DMAcPA is then added to the solution at the concentration of 1.0 - 7.0 mg/ml. The doped solution was spin-coated on the substrate for 30 s at 4000 rpm in a nitrogen filled glove box. The DMAcPA coated substrate is then annealed for 10 min at 100 ℃.
General Information
| CAS Number | 2971088-37-4 |
|---|---|
| Chemical Formula | C19H22Br2NO3P |
| Molecular Weight | 503.16 g/mol |
| Absorption* | λmax (n.a.) |
| Fluorescence | λem (n.a.) |
| HOMO/LUMO | HOMO = 5.98 eV, LUMO = 2.33 eV |
| Synonyms | (4-(2,7-Dibromo-9,9-dimethylacridin-10(9H)-yl)butyl)phosphonic acid |
| Classification or Family | Carbazole derivatives, Self-assembly monolayers, Hole transport layer, Hole extraction layer, p-i-n Perovskite solar cells, Organic photovoltaics |
Product Details
| Purity | > 98% (HPLC) |
|---|---|
| Melting Point | Td = 217.8 °C |
| Appearance | Pale grey powder |
Chemical Structure
MSDS Documentation
Literature and Reviews
- Inverted perovskite solar cells using dimethylacridine-based dopants, Q. Tan et al., Nature, 620, 545–551 (2023); DOI: 10.1038/s41586-023-06207-0.
-
Progress and issues in p-i-n type perovskite solar cells, Decarbon, 3, 100025 (2024); DOI: 10.1016/j.decarb.2023.100025.
- Self-assembled molecules as selective contacts for efficient and stable perovskite solar cells, W. Li et al., Mater. Chem. Front., 8, 681-699 (2024); DOI: 10.1039/D3QM01017A.
