Dimethylammonium Iodide (DMAI)
CAS Number 51066-74-1
Materials, Perovskite Materials, Perovskite Precursor MaterialsSpecifications | MSDS | Literature and Reviews
Dimethylammonium iodide (DMAI) is the precursor for the DMAPbI3 2H-perovskite structure. Unlike its MAPbI3 analog, it is stable under ambient conditions for several months [1]. DMAPbI3 has a relatively large optical gap (Eg ∼ 2.59 eV), indicating the conductivity of such material is ionic, instead of being electronic.
Like other alkylammonium halides, dimethylammonium iodide is also used as an additive to adjust the structures of the perovskites to achieve desired physical properties.
General Information
| CAS number | 51066-74-1 |
| Chemical formula | C2H8IN |
| Molecular weight | 173.0 g/mol |
| Synonyms | Dimethylamine hydroiodide, N-Methylmethanaminium iodide, DMAI |
| Classification / Family | Alkylammonium halides, Perovskite precursor materials, Perovskite solar cells, Perovskite LEDs |
Product Details
| Purity | >99.5% |
| Melting point | 153.85 °C |
| Color | Powder/crystals |
Chemical Structure
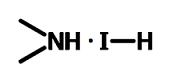
MSDS Documentation
Dimethylammonium iodide MSDS sheet
Literature and reviews
- Phase Transition, Dielectric Properties, and Ionic Transport in the [(Ch2)2NH2]PbI3 Organic–Inorganic Hybrid with 2H-Hexagonal Perovskite Structure, A. García- Fernández et al, Inorg. Chem., 2017, 56 (9), 4918–4927 (2017); DOI: 10.1021/acs.inorgchem.6b03095.
- Organic–Inorganic Perovskites: Structural Versatility for Functional Materials Design, B. Saparov et al., Chem. Rev., 116 (7), 4558–4596 (2016); DOI: 10.1021/acs.chemrev.5b00715.
- Phase Transition and Cationic Motion in a Metal–Organic Perovskite, Dimethylammonium Zinc Formate [(Ch2)2NH2][Zn(HCOO)3], T. Asaji et al., J. Phys. Chem. C, 117 (19), 10185–10190 (2013); DOI: 10.1021/jp402148y.
