Syringe Pump User Manual

Contents
- Overview
- EU Declaration of Conformity (DoC)
- Safety
- Warning
- Use of Equipment
- Hazard Icons
- General Hazards
- Power Cord Safety
- Servicing
- Health and Safety - Operation
- Health and Safety - Servicing
- Equipment Purpose
- Unpacking
- Specifications
- System Components
- Installation
- Operation
- Maintenance
- Troubleshooting
1. Overview
Syringe pumps are used in the field of scientific research to provide a reliable and repeatable method for moving liquids. Typically, the most common use of a syringe pump is to dispense a liquid at a known rate. This can be for use in processes such as pre-metered coating, automated systems, chemical synthesis, microfluidics, and flow chemistry. Other uses of syringe pumps include the withdrawal of liquids from a system for processes such as automated sample taking, and fluid level adjustment. By combining both infusion and withdrawal from syringes it is possible to use a syringe pump for an even wider array of applications including continuous flow of solutions, solution mixing, and the emulsification of solutions
A syringe pump works by fixing the barrel of the syringe to the body of the equipment while at the same time fixing the plunger to a moving platform. This movement is achieved via the use of a high accuracy stepper motor where the distance moved by the platform is well known and characterized. By knowing the internal diameter of the syringe being used it is possible to work out the volume displaced when the platform moves. The Ossila Syringe Pump provides you with a simple easily programmable system, where each program can have up to 99 different steps in which the rate, volume, and/or time of infusion/withdrawal can be chosen. By selecting the correct diameter syringe, it is possible to achieve flow rates as low as nanolitres per minute or as high as millilitres per second.
1.1 Applications
- Solution dispensing
- Solution mixing
- Emulsification
- Metered coatings
- Solution quenching
- Electrospinning
- Electrospraying
- Microfluidics
- Flow chemistry
2. EU Declaration of Conformity (DoC)
We
Company Name: Ossila BV
Postal Address: Biopartner 3 Building, Galileiweg 8
Postcode: 2333 BD Leiden
Country: The Netherlands
Telephone Number: +31 (0)718 081020
Email Address: info@ossila.com
declare that the DoC is issued under our sole responsibility and belongs to the following product:
Product: Single Syringe Pump (L2003S1), Dual Syringe Pump (L2003D1)
Serial Number: L2003S1 - xxxx, L2003D1
Object of Declaration
Single Syringe Pump (L2003S1), Dual Syringe Pump (L2003D1)
The object of declaration described above is in conformity with the relevant Union harmonization legislation:
Machinery Directive 2006/42/EC
EMC Directive 2014/30/EU
RoHS Directive 2011/65/EU
The folowing harmonized standards and technical specifications have been applied:
BS EN ISO 12100:2010 Safety of machinery - General principles for design - Risk assessment and risk reduction
Signed:

Name: Dr James Kingsley
Place: Leiden
Date: 16/11/2021
| Декларация | за съответствие на ЕС |
|---|---|
| Производител | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Декларира с цялата си отговорност, че посоченото оборудване съответства на приложимото законодателство на ЕС за хармонизиране, посочено на предходната(-ите) страница(-и) на настоящия документ. | |
| [Čeština] | Prohlášení o shodě EU |
|---|---|
| Výrobce | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Prohlašujeme na vlastní odpovědnost, že uvedené zařízeni je v souladu s příslušnými harmonizačními předpisy EU uvedenými na předchozích stranách tohoto dokumentu. | |
| [Dansk] | EU-overensstemme lseserklærin g |
|---|---|
| Producent | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Erklærer herved, at vi alene er ansvarlige for, at det nævnte udstyr er i overensstemmelse med den relevante EUharmoniseringslovgivning, der er anført på den/de foregående side(r) i dette dokument. | |
| [Deutsch] | EU-Konformitätserklärung |
|---|---|
| Hersteller | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Wir erklären in alleiniger Verantwortung, dass das aufgeführte Gerät konform mit der relevanten EUHarmonisierungsgesetzgebung auf den vorangegangenen Seiten dieses Dokuments ist. | |
| [Eesti keel] | ELi vastavusavaldus |
|---|---|
| Tootja | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Kinnitame oma ainuvastutusel, et loetletud seadmed on kooskõlas antud dokumendi eelmisel lehelküljel / eelmistel lehekülgedel ära toodud asjaomaste ELi ühtlustamise õigusaktidega. | |
| [Ελληνικά] | Δήλωση πιστότητας ΕΕ |
|---|---|
| Κατασκευαστής | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Δηλώνουμε υπεύθυνα όn ο αναφερόμενος εξοπλισμός συμμορφώνεται με τη σχεnκή νομοθεσία εναρμόνισης της ΕΕ που υπάρχει σnς προηγούμενες σελίδες του παρόντος εγγράφου. | |
| [Español] | Declaración de conformidad UE |
|---|---|
| Fabricante | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Declaramos bajo nuestra única responsabilidad que el siguiente producto se ajusta a la pertinente legislación de armonización de la UE enumerada en las páginas anteriores de este documento. | |
| [Français] | Déclaration de conformité UE |
|---|---|
| Fabricant | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Déclarons sous notre seule responsabilité que le matériel mentionné est conforme à la législation en vigueur de l'UE présentée sur la/les page(s) précédente(s) de ce document. | |
| [Hrvatski] | E.U izjava o sukladnosti |
|---|---|
| Proizvođač | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Izjavljujemo na vlastitu odgovornost da je navedena oprema sukladna s mjerodavnim zakonodavstvom EU-a o usklađivanju koje je navedeno na prethodnoj(nim) stranici(ama) ovoga dokumenta. | |
| [Italiano] | Dichiarazione di conformità UE |
|---|---|
| Produttore | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Si dichiara sotto la propria personale responsabilità che l'apparecchiatura in elenco è conforme alla normativa di armonizzazione UE rilevante indicata nelle pagine precedenti del presente documento. | |
| [Latviešu] | ES atbils tības deklarācija |
|---|---|
| Ražotājs | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Ar pilnu atbilclību paziņojam, ka uzskaitītais aprīkojums atbilst attiecīgajiem ES saskaņošanas tiesību aktiem, kas minēti iepriekšējās šī dokumenta lapās. | |
| [Lietuvių k.] | ES atitikties deklaracija |
|---|---|
| Gamintojas | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| atsakingai pareiškia, kad išvardinta įranga atitinka aktualius ES harmonizavimo teisės aktus, nurodytus ankstesniuose šio dokumento | |
| [Magyar] | EU-s megfelelőségi nyilatkozat |
|---|---|
| Gyártó | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Kizárólagos felelösségünk mellett kijelentjük, hogy a felsorolt eszköz megfelel az ezen dokumentum előző oldalán/oldalain található EU-s összehangolt jogszabályok vonatkozó rendelkezéseinek. | |
| [Nederlands] | EU-Conformiteitsverklaring |
|---|---|
| Fabrikant | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Verklaart onder onze uitsluitende verantwoordelijkheid dat de vermelde apparatuur in overeenstemming is met de relevante harmonisatiewetgeving van de EU op de vorige pagina('s) van dit document. | |
| [Norsk] | EU-samsvarserklæ ring |
|---|---|
| Produsent | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Erklærer under vårt eneansvar at utstyret oppført er i overholdelse med relevant EU-harmoniseringslavverk som står på de(n) forrige siden(e) i dette dokumentet. | |
| [Polski] | Deklaracja zgodności Unii Europejskiej |
|---|---|
| Producent | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Oświadczamy na własną odpowiedzialność, że podane urządzenie jest zgodne ze stosownymi przepisami harmonizacyjnymi Unii Europejskiej, które przedstawiono na poprzednich stronach niniejszego dokumentu. | |
| [Por tuguês] | Declaração de Conformidade UE |
|---|---|
| Fabricante | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Declara sob sua exclusiva responsabilidade que o equipamento indicado está em conformidade com a legislação de harmonização relevante da UE mencionada na(s) página(s) anterior(es) deste documento. | |
| [Română] | Declaraţie de conformitate UE |
|---|---|
| Producător | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Declară pe proprie răspundere că echipamentul prezentat este în conformitate cu prevederile legislaţiei UE de armonizare aplicabile prezentate la pagina/paginile anterioare a/ale acestui document. | |
| [Slovensky] | Vyhlásenie o zhode pre EÚ |
|---|---|
| Výrobca | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Na vlastnú zodpovednosť prehlasuje, že uvedené zariadenie je v súlade s príslušnými právnymi predpismi EÚ o harmonizácii uvedenými na predchádzajúcich stranách tohto dokumentu. | |
| [Slovenščina] | Izjava EU o skladnosti |
|---|---|
| Proizvajalec | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| s polno odgovornostjo izjavlja, da je navedena oprema skladna z veljavno uskladitveno zakonodajo EU, navedeno na prejšnji strani/prejšnjih straneh tega dokumenta. | |
| [Suomi] | EU-vaatimustenm ukaisuusvakuutus |
|---|---|
| Valmistaja | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Vakuutamme täten olevamme yksin vastuussa siitä, että tässä asiakirjassa luetellut laitteet ovat tämän asiakirjan sivuilla edellisillä sivuilla kuvattujen olennaisten yhdenmukaistamista koskevien EU-säädösten vaatimusten mukaisia. | |
| [Svenska] | EU-försäkran om överensstämmelse |
|---|---|
| Tillverkare | Ossila BV, Biopartner 3 building, Galileiweg 8, 2333 BD Leiden, NL. |
| Vi intygar härmed att den utrustning som förtecknas överensstämmer med relevanta förordningar gällande EUharmonisering som fmns på föregående | |
3. Safety
3.1 Warning
3.2 Use of Equipment

CAUTION: For research use only. Not for clinical use on patients.
The Ossila Syringe Pump is designed to be used as instructed, it is intended to be used under the following conditions:
- Indoors in a laboratory environment (pollution degree 2).
- Altitudes up to 2000 m.
- Temperatures of 5°C to 40°C; maximum relative humidity of 80% up to 31°C.
The Syringe Pump is supplied with a 24V/2A power adapter with a power cord for the country of purchase, in accordance with European Commission regulations and British Standards. Use of any other electrical power cables, adaptors or transformers is not recommended.
3.3 Hazard Icons
The symbols shown in Table 3.1 can be found at points throughout the manual. Note each warning before attempting any associated operations.
Table 3.1 Hazard warning labels used in this manual.
| Symbol | Associated Hazard |
|---|---|
 |
General warning or caution, which accompanying text will explain |
 |
Electrical shock |
 |
Pinch point or entanglement hazard |
3.4 General Hazards
Before installing or operating the Syringe Pump, there are several health and safety precautions which must be followed and executed to ensure safe installation and operation.
WARNING: Improper handling when operating or servicing this equipment can result in serious injury. Read this manual before operating or servicing this equipment.

CAUTION: During operation, the movement of the plunger clamp and the rotation of the lead screw/coupler could result in a pinch point hazard and/or an entanglement hazard. Caution should be taken whenever the system is in operation.
3.5 Power Cord Safety

Emergency power disconnect options: Use the power cord as a disconnecting method and remove it from the power source. To facilitate disconnect, make sure the power outlet for this cord is readily accessible to the operator.
3.6 Servicing
If servicing is required, please return the unit to Ossila Ltd The warranty will be invalidated if:
- Modification or service has taken place by anyone other than an Ossila engineer.
- The unit has been subjected to chemical damage through improper use.
- The unit has been operated outside the usage parameters stated in the user documentation associated with the unit.
- The unit has been rendered inoperable through accident, misuse, contamination, improper maintenance, modification, or other external causes.
3.7 Health and Safety - Operation

Pinch point and entanglement hazards are present during operation of the syringe pump. As some precaution users should avoid handling or leaning over the equipment during operation in order to avoid possible crushing or entanglement of hair and/or clothing.
3.8 Health and Safety - Servicing

Service or installation work that includes integrating electrical components should be performed by an Ossila engineer only. Never alter the wiring of any purchased equipment. If changes are made, such alterations may damage the equipment, cause injury or death. At the very least, such alterations may void your equipment’s warranty.
4. Equipment Purpose
The Ossila Syringe Pump has been designed to provide users with a tool to move volumes of fluids accurately and repeatedly at specified rates. The equipment can be used in a wide variety of experiments where accurate dispense volumes and/or dispense rates are required. In order to achieve this, the system uses a stepper motor to enable movement of specified distances. This is done by securing the body of the syringe to the frame of the syringe pump while at the same time securing the syringe plunger to the moving holder. The internal diameter of the syringe can be used to determine the volume displaced by the movement of the stepper motor and plunger. By choosing the appropriate syringe size, a wide range of dispense rates can be achieved.
The exact syringe types, deposition rates, deposition volumes, and solutions used are up to the user to choose and will vary depending upon the type of experiment being undertaken. For more information on uses please see the operating instructions and guidelines, for more information on the dispensing limits please see the specifications.
5. Unpacking
5.1 Packing List
The standard items included with the Syringe Pump are:
- The Syringe Pump unit
- Universal power adapter
5.2 Damage Inspection
Examine the components for evidence of shipping damage, if damage has occurred, please contact Ossila directly for further action. The shipping packaging will come with a shock indicator to show if there has been any mishandling of the package during transportation
6. Specifications
The specifications for the syringe pump software are shown in Table 6.1, and the hardware specifications are shown in Table 6.2.
Table 6.1. Ossila Syringe Pump software specifications
| Number of Programmes | 10 |
|---|---|
| Steps per Programme | 99 |
| Syringe Control | Independent Control |
| Syringe Size Settings | 5 pre-sets, 1 customizable diameter |
| Force Tuning | 5% to 100% (5% increments) |
| Minimum Syringe Size | 0.5μl |
| Memory | Non-Volatile Memory |
Table 6.2. Ossila Syringe Pump hardware specifications
| Syringes | 1 (L2003S1); 2(L2003D1) |
|---|---|
| Operation mode | Infusion and withdrawal |
| Rate accuracy | ±3% |
| Drive motor | 1.8° Stepper motor |
| Max linear force | 500N at 100% force |
| Motor drive controller | Microprocessor with 1/64 microstepping |
| Number of microsteps per revolution | 12,800 |
| Screw pitch | 1mm |
| Distance per microstep | 78nm |
| Maximum step rate/distance | 27 μstep.min-1; 5mm.s-1 |
| Minimum step rate/distance | 27sec.μstep-1; 12μm.s-1 |
| Maximum syringe size | 50 (60)ml |
| Safety | Limit switches; crash sensing |
| Power | 24 V DC; 2.0 A |
| Power supply Input voltage range | 100 - 240VAC; 50/60Hz; 50VA |
| Operating temperature/humidity | 5°C to 40°C; Up to 80% RH @ 31°C |
| Overall dimensions |
Width: 330mm Height: 140mm Depth: 260mm |
7. System Components
The Syringe Pump (L2003S1/L2003D1) comprises two items:
- Syringe Pump unit (Figure 7.1)
- 24 V mains power supply adapter (Figure 7.2)
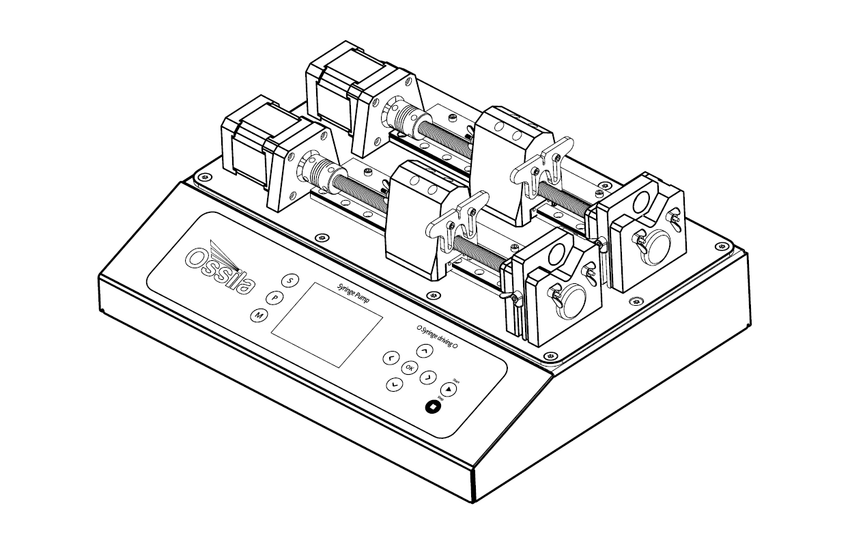
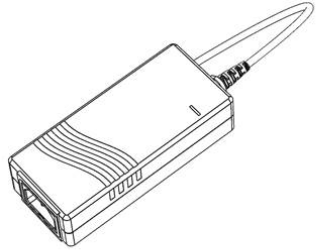
The Syringe Pump unit is powered from a 24 V, 2 A mains power supply adapter. This low voltage supply is convenient and reduces the health and safety risks involved.
8. Installation
- Place the unit on a solid, level work surface.
- Ensure the area is free from vibrations, temperature extremes and highly flammable or explosive materials.
- Before plugging in the Syringe Pump, ensure the power switch on the unit is switched to the ‘0’ position (off).
- Connect the power supply first to the mains outlet and second to the Syringe Pump unit.
- See Figure 8.1 for connecting the Syringe Pump to the power supply cable.
- Set the Syringe Pump power switch to the ‘1’ position to turn on.

9. Operation
9.1 Syringe Pump Schematic
A top-down view of the Syringe Pump is shown in Figure 9.1 with all the relevant components highlighted.

9.2 Attaching a Syringe to the System
When using the Syringe Pump the following procedures should be done while attaching the syringe to the system to achieve the best results.
- The solution should be preloaded into the syringe; make sure all air bubbles are removed from the syringe before attaching to the system.
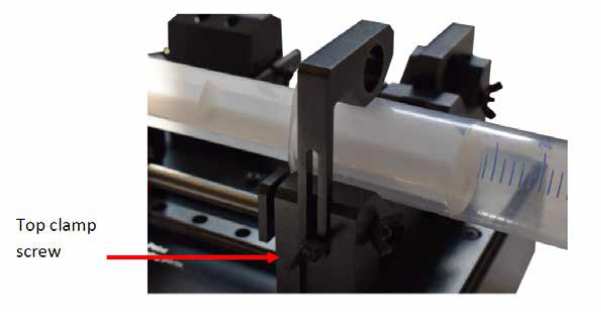
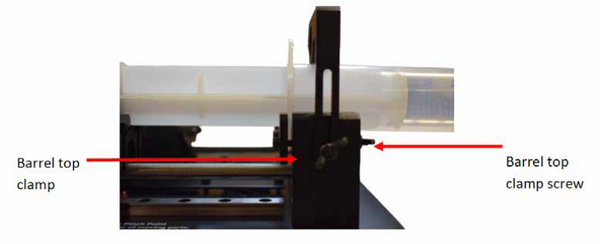
- The syringe barrel should be placed flat into the groove on the syringe holder, and the barrel top should be placed inside the clamping mechanism (see Figure. 9.2). The top clamping arm should be lowered onto the top of the syringe and locked into place by tightening the wing nut on the side of the syringe holder. The barrel top should be clamped into place by tightening the wing nut screws as the opposite end of the syringe holder.

- Enter manual mode on the syringe and drive the plunger holder till it rests against the end of the plunger. Slide the end of the plunger into the clamping mechanism and tighten, making sure it is parallel with the barrel of the syringe.
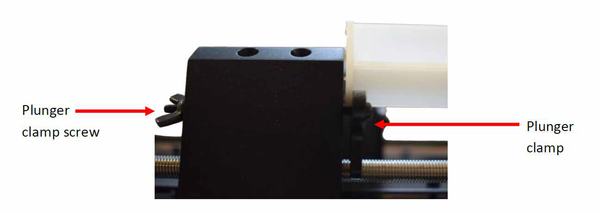
- Connect any additional tubing or fixtures required for your experiment. Using the manual mode preload the fixtures and tubing with your solution.
9.3 User Interface
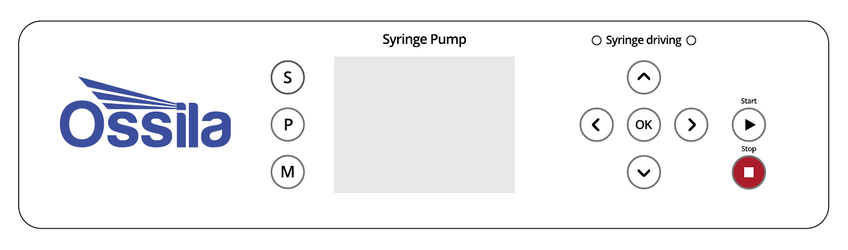
Table 9.1. Operational buttons and their associated functions
| Button | Function |
|---|---|
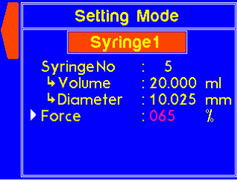
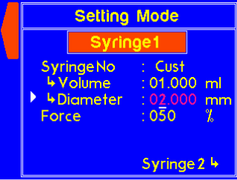
| S (Settings) | Enters the Settings menu where syringe diameter, and maximum force can be set |
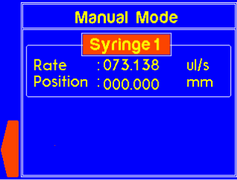
| M (Manual) | Enters Manual mode where the plunger holder can be moved |
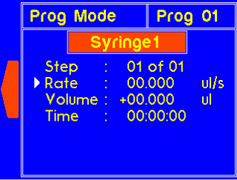
| P (Program) | Enters the Program mode where saved programs can be selected and/or edited |
| Navigates ‘Up’ through menus; increases selected values by 1; increases unit size | |
| Navigates ‘Down’ through menus; decreases selected values by 1; decreases unit size | |
| Navigates ‘Right’ through menus | |
| Navigates ‘Left’ through menus | |
| Press to select, edit, or accept changes | |
| Stop current running program | |
| Run currently selected program |
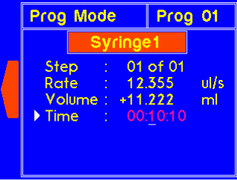
9.4 Program Operation
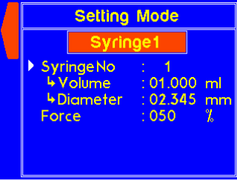
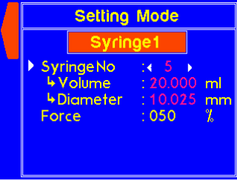
9.4.1 MODEL: L2003S1 (Single Syringe Pump)

WARNING! Pinch points and entanglement hazards!
The rotation of the lead screw/coupler and the movement of the plunger holder present entanglement and pinch hazards. Care should be taken when the system is running.
|
 |
|
 |
|
 |
|
 |
|
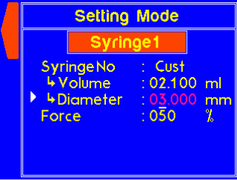
A warning will appear if the diameter is not set for a custom syringe. |
 |
|
A warning will appear if the volume is not set for a custom syringe. |
 |
|
 |
|
 |
|
 |
|
 
|
|
 |
|
 |
Note: The dispense time for the step will automatically update to account for the new rate. |
 |
|
 |
|
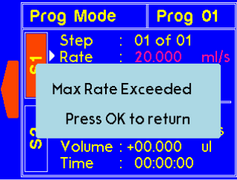
A warning will appear if the set rate is higher than allowed for the syringe size. The value will be recalculated and set to max rate for the particular syringe. |
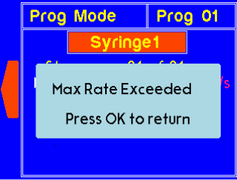 |
Note: The dispense time for the step will automatically update to account for the new rate. |
 |
|
 |
|
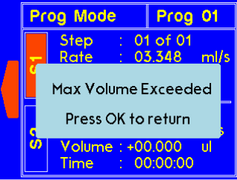
A warning will appear if the set volume higher than allow for the syringe size. The value will be recalculated and set to max volume for the syringe. |
 |
|
 |
The dispense volume for the step will automatically update to account for the new dispense time setting. A warning will appear if the volume is larger than allowed for the syringe size. The value will be recalculated to the correct volume for the syringe. |
 |
|
 |
|
 |
|
 |
|
The Ossila Syringe Pump is equipped with a safety warning. The System temperature is monitored while the system is On. In the case of high temperatures, a warning will appear to notify the user. |
 
|
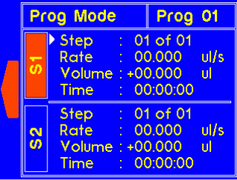
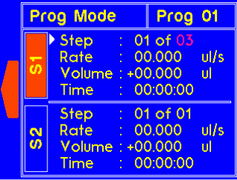
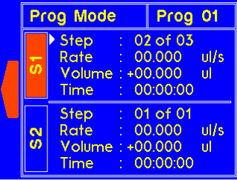
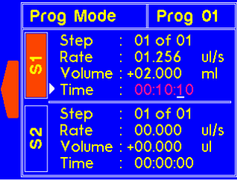
9.4.2 MODEL: L2003D1 (Dual Syringe Pump)

WARNING! Pinch points and entanglement hazards!
The rotation of the lead screw/coupler and the movement of the plunger holder present entanglement and pinch hazards. Care should be taken when the system is running.
|
 |
|
 
|
|
 
|
|
 |
|
A warning will appear if the diameter is not set for custom syringe. |
 |
|
A warning will appear if the volume is not set for custom syringe. |
 |
|
 
|
|
 
|
|
 |
|
 
|
|
 |
|
 |
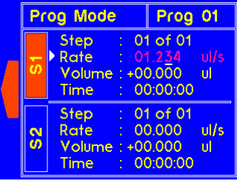
The dispense time for the step will automatically update to account for the new rate. |
 |
|
 |
|
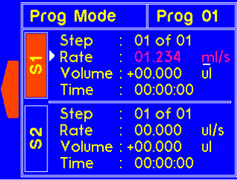
A warning will appear if the set rate higher than allow for the syringe size. The value will be recalculated and set to max rate for the particular syringe. |
 |
The dispense time for the step will automatically update to account for the new rate. |
 |
|
 |
|
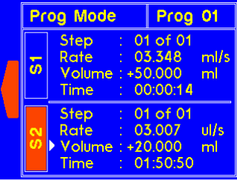
A warning will appear if the set volume is larger than the syringe size can allow. The value will be recalculated and set to the max volume for the syringe. |
 |
|
 |
The dispense volume for the step will automatically update to account for the new dispense time setting. A warning will appear if the volume is larger than the syringe size allows. The value will be recalculated to the correct volume for the syringe. |
 |
|
 |
|
|
|
 |
|
 |
|
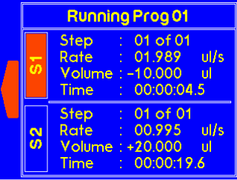 |
|
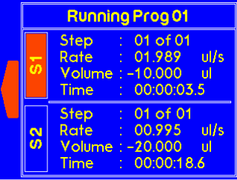 |
|
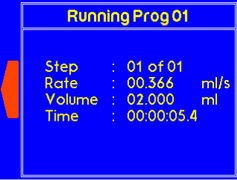
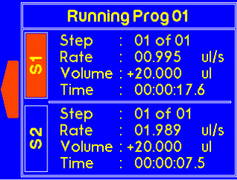
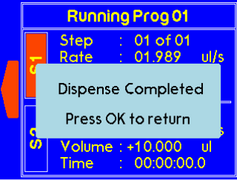
Once the timer is finished, a warning will appear to alert the suer that dispensing is complete. |
 |
|
If the one of the syringes completes dispensing, the “complete” message will pop up to notify the user. |
 
|
|
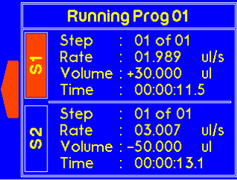
While the system is running, the user can stop one of the syringes by pressing the “Up” or “Down” buttons. Press “OK” to stop the selected syringe. |
 
|
|
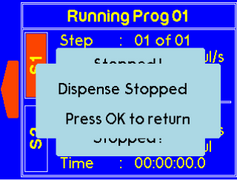
If both syringes are stopped by the user, the message will appear as follow. |
 |
|
To stop both syringes simultaneously, press the “Stop” button. The message will appear as follow. |
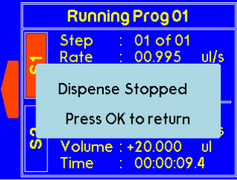 |
|
The Ossila Syringe Pump is equipped with a safety warning. The System temperature is monitored while the system is On. In the case of high temperatures, a warning will appear to notify the user. |
 |
|
The system is also equipped with safety switches in case of software malfunction. There are two switches to safeguard forward and reverse movement. In the event of either switch being pressed, the following warning will appear: |
 
|
10. Maintenance
The rail guide and the lead screw should be sparingly lubricated periodically; every 200 hrs of usage. The rail guide and the lead screw should be lubricated with super lube synthetic grease provided with the pump.
To clean the exterior surfaces, use a lint-free cloth to remove loose dust. Use care to avoid scratching the clear display window. For more efficient cleaning, use a soft cloth dampened (not soaked) with water, an aqueous solution of 75% isopropyl alcohol, or a mild detergent.
11. Troubleshooting
Table 11.1. Troubleshooting guidelines for the Ossila Syringe Pump
| Problem | Possible Cause | Action |
|---|---|---|
| No power/display | The power switch on the unit is in the OFF position. | Check the connection and ensure the power is turned ON. |
| The power supply may not be connected properly. | Ensure the unit is firmly plugged in to the power supply and the plug is firmly connected to both the adapter and the working power socket. | |
| The fuse on the rear panel has blown. | Ensure the unit is unplugged. Check the fuse on the rear panel. If it has blown, replace with a suitably rated 1A slow blow fuse. | |
| If the unit was supplied with a transformer, it may be faulty. | Contact Ossila for a replacement transformer. | |
| Fault on circuit board. | If all the above causes have been considered, there may be a fault on the control board. Please contact Ossila for information | |
| Motor Stalled (Syringe Pump) | Syringe has hit first safety switch | Syringe plunger hitting the bottom / a kink in the tubing (occlusion) / Syringe plunger binding / Low force setting |
| Crash Warning | The secondary safety switch has accidentally been triggered | Turn the unit off and on again to see if the warning remains |
| The syringe holder is triggering the secondary safety switch | Rotate the lead screw by hand to move the syringe holder closer to the center of travel | |
| Internal fault in the secondary safety switch | If all the above has been considered there may be a fault with the secondary safety switch; contact Ossila for more information |