DPP-BO-2ThBr
CAS Number 1224709-68-5
Chemistry Building Blocks, Dibromo Monomers, Heterocyclic Building Blocks, Materials, MonomersDPP-BO-2ThBr, for the synthesis of low band-gap semiconducting polymers
High purity monomer for applications in OFETs and solar cells
Specifications | MSDS | Literature and Reviews
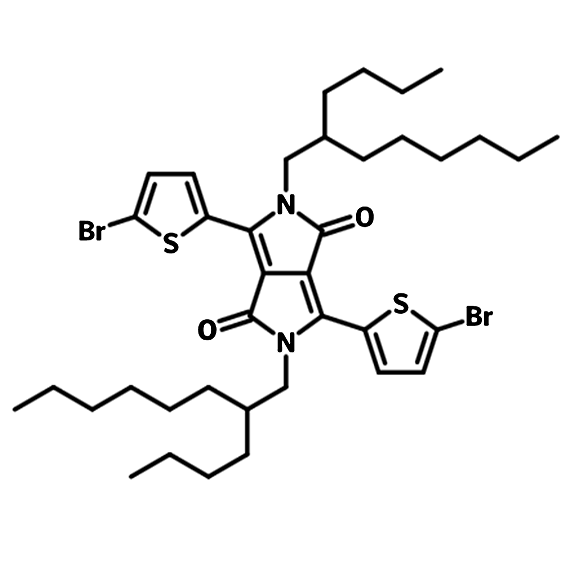
3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-butyloctyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione (DPP-BO-2ThBr), CAS number 1224709-68-5, is used for the synthesis of low band-gap polymer semiconductors (such as PBDTT-DPP) for OPV and perovskite solar cell applications.
DPP-BO-2ThBr is also used as an intermediate for the synthesis of small molecules, and as an electron-donor material for applications in OFETs and solar cells.
This product has been used in our own lab for the synthesis of PBDTT-DPP.
General Information
| CAS Number | 1224709-68-5 |
| Chemical Formula | C38H54Br2N2O2S2 |
| Molecular Weight | 794.79 g/mol |
| Synonyms | 3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-butyloctyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione |
| Classification / Family | Pyrroles, Pyrrolopyrrole-dione (DPP), Thiophenes, Acceptor, Organic semiconducting materials, Semiconductor synthesis, Building blocks |
Chemical Structure
Product Details
| Purity | >98% |
| Melting Point | N/A |
| Appearance | Dark red/purple solid |
MSDS Documentation
Literature and Reviews
- A benzothiadiazole end capped donor–acceptor based small molecule for organic electronics, P. Sonar et al., Phys. Chem. Chem. Phys., 15, 17064-17069 (2013); DOI: 10.1039/C3CP52929K.
- Tandem polymer solar cells featuring a spectrally matched low-bandgap polymer, L. Dou et al., Nat. Photonics 6, 180 (2012); DOI: 10.1038/NPHOTON.2011.356.
- High-performance multiple-donor bulk heterojunction solar cells, Y. Yang et al., Nat. Photonics 9, 190–198 (2015); DOI:10.1038/nphoton.2015.9.

 DPP-BO-2ThBr MSDS Sheet
DPP-BO-2ThBr MSDS Sheet