3-Hexylthiophene
CAS Number 1693-86-3
Chemistry Building Blocks, Heterocyclic Building Blocks, Materials, Monomers3-Hexylthiophene, for the synthesis of P3HT
High-quality and high-purity monomer available online
Specifications | MSDS | Literature and Reviews
3-Hexylthiophene, CAS number 1693-86-3, is the intermediate for the synthesis of poly(3-hexylthiophene), referred as P3HT. To date, it is the most studied polymer for polymer solar cells. The efficiency of a P3HT/PCBM solar cell is typically 4-5 %, but with new fullerene materials developed to closely match the energy levels of P3HT (HOMO 5.0 eV, LUMO 3.0 eV), device performances have been pushed to 6.5% [1].
The synthesis of P3HT is relatively easy and short, only a 3-4 step synthesis is required [2, 3, 4]. The challenge of P3HT is that it only absorbs a narrow band of solar spectrum, so it has little room for improvement in terms of performance efficiency.
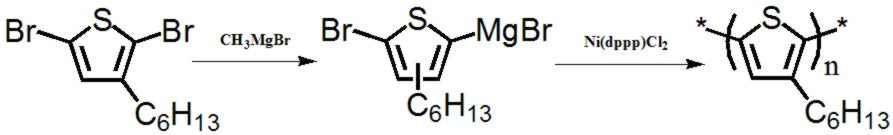
A synthesis precusor
For poly(3-hexylthiophene) (P3HT)
Hexylthiophene building block
For semiconductors, OFETs, and solar cells
Worldwide shipping
Quick and reliable shipping
High purity
>99% High purity
General Information
| CAS Number |
1693-86-3 |
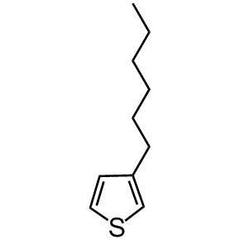
| Chemical Formula | C10H16S |
| Molecular Weight | 168.30 g/mol |
| Synonyms | 1-(Thien-3-yl)hexane 3-n-Hexylthiophene |
| Classification / Family | Monomers, Building blocks, Thiophene, Heterocycles, Chemical synthesis for low band gap polymers, Intermediates for OFETs, OLED, Organic Photovoltaics, Polymer solar cells |
Chemical Structure
Product Details
| Purity | 99% |
| Boiling Point | 65 °C at 0.45 mmHg (lit.) 299 °C at 760 mmHg (1 atm, lit.) |
| Density | 0.936 g/cm3 |
| Appearance | Colourless/pale yellow liquid |
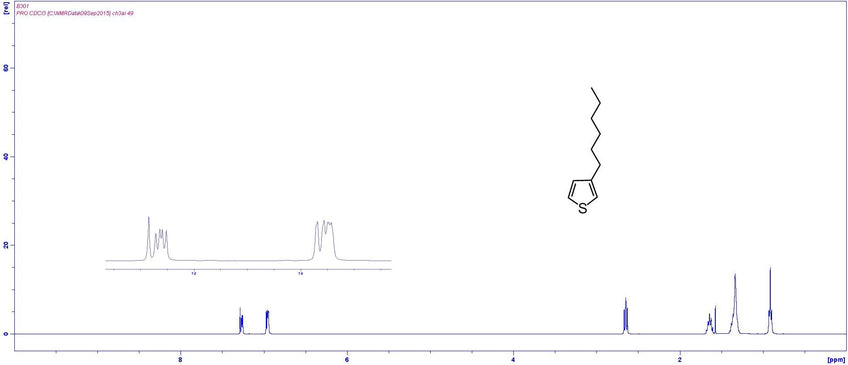
NMR Characterisation
MSDS Documentation
Literature and Reviews
- 6.5% Efficiency of Polymer Solar Cells Based on poly(3‐hexylthiophene) and Indene‐C60 Bisadduct by Device Optimization, G. Zhao et al., Adv. Mater., 22, 4355–4358 (2010).
- Regiocontrolled Synthesis of Poly(3-alkylthiophenes) Mediated by Rieke Zinc: Their Characterization and Solid-State Properties, Chen et. al., J. Am. Chem. Soc., 117 (1), pp 233–244 (1995).
- A Simple Method to Prepare Head-to-Tail Coupled, Regioregular Poly(3-alkylthiophenes) Using Grignard Metathesis., R. S. Loewe et al., Adv. Mater., 11: 250–253 (1999)

 3-Hexylthiophene MSDS sheet
3-Hexylthiophene MSDS sheet