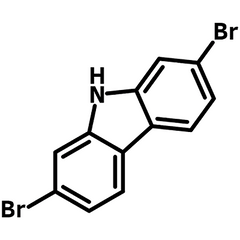
2,7-Dibromocarbazole
CAS Number 136630-39-2
Chemistry Building Blocks, Heterocyclic Building Blocks, Materials, Monomers2,7-Dibromo-9H-carbazole, high quality and high purity monomer
Popular candidate for the application of organic electronics
Specifications | MSDS | Literature and Reviews
2,7-dibromocarbazole (CAS number 136630-39-2), also known as 2,7-dibromo-9H-carbazole, is a building block for the synthesis of small molecules of carbazole series or carbazole back-boned polymers in the application of OFETs, OLEDs, PLEDs and OPVs.
Carbazole derivatives, small molecules or poly(2,7-carbazole)s, have been one of the most popular candidates for the application of organic electronics due to their well-known good electron-donating abilities and chemical stability comparing with fluorene units. Also, the functional group on the 9-position makes them chemically available to be easily adjustable to different aromatic or alkyl chains to allow solubility for devices film morphology or even further tuning the energy levels of such compounds.
One of the most popular products made available from 2,7-dibromocarbazole is PCDTBT, a well-known polymer semiconductor used for polymer solar cells. A good example for adjusting the solubility of the carbazole compounds by using 2,7-dibromocarbazole as the starting materials is the synthesis of 2,7-dibromo-9-heptadecanylcarbazole.
Carbazole building block
for the synthesis of OLED and organic photovoltaic materials
Worldwide shipping
Quick and reliable shipping
Capped with bromides
for facile coupling reactions
High purity
>99% Purity
General Information
| CAS Number | 136630-39-2 |
| Chemical Formula | C12H7Br2N |
| Molecular Weight | 325.0 g/mol |
| Synonyms | 2,7-Dibromo-9H-carbazole |
| Classification / Family | Monomers, Building blocks, Carbazoles, Halogenated heterocycles, Chemical synthesis for low band gap polymers, Intermediates for semiconducting polymers |
Chemical Structure
Product Details
| Purity | >99% |
| Melting Point | 226 °C - 230 °C |
| Appearance | Off-white powder |
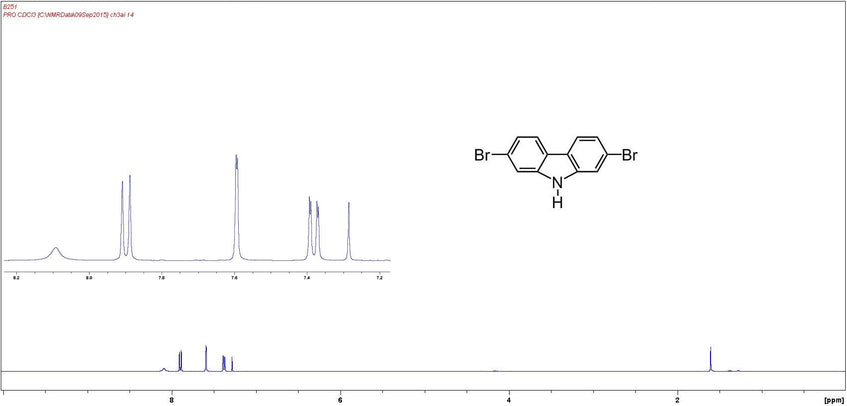
Characterization by 1H-NMR (example)
MSDS Documentation
2,7-Dibromocarbazole MSDS sheet
Literature and Reviews
- Solution-processed blue phosphorescent OLEDs with carbazole-based polymeric host materials, F. Dumur et al., Org. Electronics 25, 21–30 (2015)
- Synthesis, Characterization, and Use as Emissive Layer of White Organic Light Emitting Diodes of the Highly Isotactic Poly(N-pentenyl-carbazole), A. Botta et al., poly. Composites, 1110-1117 (2015)
- A low bandgap carbazole based small molecule for organic solar cells, M. Li, et al., Org. Electronics 24, 89-95 (2015)
