Titanium(IV) Sulfide (TiS2) Powder and Crystal
CAS Number 12039-13-3
2D Materials, Low Dimensional Materials, Materials, Transition Metal Chalcogenides (TMCs),Low price, high purity 2D metal titanium(IV) sulfide powder and crystals
For the development of next-generation electronics, optoelectronics, and nanotechnology
Technical Data | MSDS | Structure | Literature and Reviews | Related Products | Resources and Support
Titanium(IV) sulfide (also known as titanium disulfide TiS2), CAS number 12039-13-3, is highly stable. It is the most lightweight and low-cost Group IV TMDC material. TiS2 is known to be a semimetal in when in bulk form with an indirect band-overlap of about 0.12 eV, and a semiconductor when strain or pressure is applied in the monolayer form. With a higher degree of overlap of the valence and conduction bands, its semi-metallic nature intensifies when compression is applied. However, the energetic overlap is lifted and a semimetal-to-semiconductor transition occurs under pressure or strains.
High Purity
≥99.999% Crystal Purity
Worldwide Shipping
Quick and reliable shipping
Low Cost
Low Cost Titanium(IV) Sulfide
Powder & Crystal
Different Forms of Titanium(IV) Sulfide
TiS2 crystallizes in an octahedral (1T) phase, which is more energetically stable compared to its hexagonal (2H) phase. Titanium(IV) sulfide has been successfully employed as an electrode material for rechargeable sodium and lithium ion batteries.
We supply low price titanium(iv) sulfide in several different forms for a range of applications.

Titanium(IV) Sulfide Powder
Can be used for preparation of titanium(IV) sulfide nanoplates and ultrathin films
Sold by weight
≥99.995% purity
From £350
Titanium(IV) Sulfide Crystals by Size
Can be used to produce single or few-layer titanium(IV) sulfide sheets via mechanical or liquid exfoliation
Small (≥10 mm2) or medium (≥25 mm2) crystals available*
≥ 99.999% purity
From £520
*Typical representative size, areas/dimensions may vary
Bulk single titanium(IV) sulfide crystal is most commonly used as sources from which single or few-layer sheets can be obtained via either mechanical or liquid exfoliation.
Titanium(IV) sulfide powder can also be used to prepare TiS2 nanosheets and nanoparticles by liquid-exfoliation (normally assisted by sonication).
Technical Data
| CAS Number | 12039-13-3 |
| Chemical Formula | TiS2 |
| Molecular Weight | 111.987 g/mol |
| Bandgap | N/A |
| Preparation | Synthetic - Chemical Vapor Transport (CVT) |
| Structure | Octahedral (1T) |
| Electronic Properties | Semimetal, Diamagnetic |
| Melting Point | N/A |
| Color | Golden yellow |
| Synonyms | Titanium disulfide, Bis(sulfanylidene)titanium |
| Classification / Family | Transition metal dichalcogenides (TMDCs), Nano-electronics, Nano-photonics, Photovoltaic, Materials science |
Product Details
| Form | Purity |
|---|---|
| Powder | ≥99.995% |
| Crystal | ≥99.999% |
Pricing Table
| Product Code | Form | Size/Weight* | Price |
|---|---|---|---|
| M2147C1 | Powder | 1 g | £350 |
| M2147A10 | Crystal | Small (≥10 mm2) | £520 ea. |
| M2147A25 | Crystal | Medium (≥25 mm2) | £850 ea. |
*Typical representative size, areas/dimensions may vary
Shipping is free for qualifying orders.
MSDS Documents
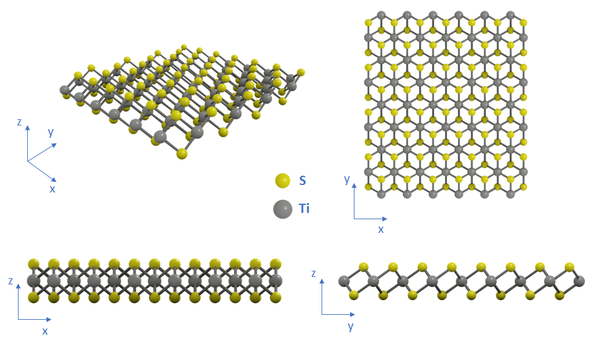
Structure of Titanium(IV) Sulfide
A single sheet of TiS2 is formed by a titanium atom layer sandwiched between two layers of sulfur atoms that are covalently bonded to the titanium atoms. Like other Group IV transition metal dichalcogenides, TiS2 crystallizes in an octahedral (1T) phase, which is more energetically stable compared to its hexagonal (2H) phase.
Literature and Reviews
- Electronic Properties and Chemical Reactivity of TiS2 Nanoflakes, C. Cucinotta et al., J. Phys. Chem. C, 119, 15707−15715 (2015); DOI: 10.1021/acs.jpcc.5b03212
- Tracking the Chemical and Structural Evolution of the TiS2 Electrode in the Lithium-Ion Cell Using Operando X‑ray Absorption Spectroscopy, L. Zhang et al., Nano Lett., 18, 4506−4515 (2018); DOI: 10.1021/acs.nanolett.8b01680.
- Room Temperature and Aqueous Solution-Processed 2D TiS2 as Electron Transport Layer for Highly Efficient and Stable Planar n-i-p Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 10 (17), 14796-14802 (2018); DOI: 10.1021/acsami.8b03225.
Related Products
We stock a wide range of 2D materials available to purchase online. Please contact us if you cannot find what you are looking for.



