PFN-I
CAS Number 1687752-60-8
Cathode Interlayer Materials (CIMs), Interface Polymers, Materials, Organic Conductors, Perovskite Interface Materials, Perovskite Materials,PFN-I, high quality active layer material for blue OLEDs
Used to improve extraction efficiencies in organic electronic devices
Overview | Specifications | MSDS | Pricing and Options | Literature and Reviews
PFN-I (CAS number 1687752-60-8) is the diiodide salt of PFN, and is a conjugated polymer electrolyte (CPE). It is commonly used as an electron-interface layer material in organic electronic devices (including OLED, OPV and perovskite solar cells) to improve extraction efficiency. PFN-I is also used as an active layer material for blue OLED devices.
Cathode interlayer molecule
For high efficient OPV applications
Improve extraction efficiency
as a conjugated polyelectrolyte
Worldwide shipping
Quick and reliable shipping
Enhance device performance
strong polar ionic pendant groups
It is believed that the device performance of polymer LEDs with bilayer cathodes (such as. PFN-I/Al), can be enhanced to levels comparable to (and even higher than) those obtained from using Ca or Ba cathodes [1].
Due to its strong polar ionic pendant groups, PFN-I is soluble in methanol and water.
| EL Polymer | Cathode | Luminance (cd m -2) | QE (%) | LE (Cd A-1) |
|---|---|---|---|---|
| MEH-PPV | Al | 6 | 0.02 | 0.02 |
| MEH-PPV | Ba/Al | 749 | 2.46 | 2.1 |
| MEH-PPV | PNF-I/Al | 773 | 2.85 | 2.4 |
General Information
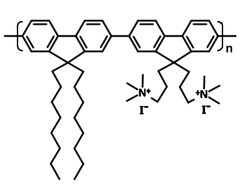
| Full name | Poly[9,9-dioctyl-9',9'-bis[3-(trimethylammonio)propyl][2,2'-bi-9H-fluorene]-7,7'-diyl iodide |
| Synonyms | PFN-iodide |
| Chemical formula | (C54H76N2)n 2I |
| CAS number | 1687752-60-8 |
| Classification/Family | Conjugated polymers, Polymer electrolyte, Polyfluorenes, Electron interface layer materials, OLED, OPV, Perovskite materials |
Chemical Structure
MSDS Documentation
Pricing
| Batch | Quantity | Price |
| M2079A | 100 mg | £280 |
|---|---|---|
| M2079A | 250 mg | £560 |
| M2079A | 500 mg | £950 |
Batch information
| Batch | Mw | Mn | PDI | Stock info |
| M2079A1 | 65 kDa | 28.3 kDa | 2.3 | Discontinued |
|---|---|---|---|---|
| M2079A2 | 150 kDa | 51.7 kDa | 2.9 | In Stock |
| M2079A3 | 62 kDa | 34.4 kDa | 1.8 | Discontinued |
Literature and References
- Efficient Electron Injection from a Bilayer Cathode Consisting of Aluminum and Alcohol-/Water-Soluble Conjugated Polymers, H. Wu et al., Adv. Mater., 16, 1826–1830 (2004), DOI: 10.1002/adma.200400067.
- Bimolecular Excited-State Electron Transfer with Surprisingly Long-Lived Radical Ions, A. A. Alsam et al., J. Phys. Chem. C 2015, 119, 21896−21903 (2015); DOI: 10.1021/acs.jpcc.5b06636.
- High-performance semi-transparent polymer solar cells possessing tandem structures, C. Chen et al., Energy. Environ. Sci., 6, 2714-2720( 2013); DOI: 10.1039/c3ee40860d.
