A ruthenium bipyridine complex
applied as a high performance dye in DCCSs
Specifications | MSDS | Literature and Reviews
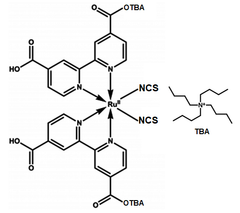
Di-tetrabutylammonium cis-bis(isothiocyanato)bis(2,2′-bipyridyl-4,4′-dicarboxylato)ruthenium(II), N719 dye (CAS number 207347-46-4), is the ammonium salt of N3 dye. With changes to increase cell voltage and solubility in polar solvents comparing with N3 dye, N719 dye is also one of the most common high performance dyes that are developed for DSSC solar cells.
N719 dye has the following features:
- Amphiphilic
- Increased electrostatic binding onto the TiO2 surface at lower pH values
- Increased stability
- Increased oxidation potential leading to deeper HOMO energy levels and enhanced stability
Ruthenium bipyridine complex
A high performance dyes used in DSSCs
Worldwide shipping
Quick and reliable shipping
Amphiphilic and stable
increased electrostatic binding to TiO2 and oxidation potential
High purity
>97%
General Information
| Full name | Di-tetrabutylammonium cis-bis(isothiocyanato)bis(2,2′-bipyridyl-4,4′-dicarboxylato)ruthenium(II) |
| Synonyms | N719 standard Dye |
| Chemical formula | C58H86N8O8RuS2 |
| Molecular weight | 1188.55 |
| CAS number | 207347-46-4 |
| HOMO / LUMO | HOMO = -6.01 eV, LUMO = -3.64 eV [1] |
| Solubility | Ethanol or acetonitrile/tert-butanol (1:1, v:v) |
| Classification / Family | Transition metal complex, Ruthenium complex, Bipyridyl ligands, Energy materials, Dye-sensitized solar cell (DSSC) materials, Donor materials, OPV materials. |
Product Details
| Purity | >97% |
| Melting point | 250 - 260 °C |
| Appearance | Brownish-red (maroon) crystalline powder |
MSDS Documentation
Literature and Reviews
- All-solid-state dye-sensitized solar cells with high efficiency, I. Chung et al., Nature, 485, 486–489 (2012); doi:10.1038/nature11067.
- Amphiphilic Ruthenium Sensitizers and Their Applications in Dye-Sensitized Solar Cells, C. Klein et al., Inorg. Chem., 43 (14), 4216–4226 (2004); DOI: 10.1021/ic049906m.
- Energy barrier at the N719-dye/CsSnI3 interface for photogenerated holes in dye-sensitized solar cells, J. Zhang et al., Sci. Reports 4, 6954 (2014); doi:10.1038/srep06954.
