2,2′:5′,2′′-Terthiophene
CAS Number 1081-34-1
Chemistry Building Blocks, Heterocyclic Building Blocks, Materials, Monomers2,2′:5′,2′′-Terthiophene (α-terthienyl)
An oligothiophene, widely used for the further synthesis of semiconducting molecules, oligomers and conjugated polymers, is also used as starting material for liquid crystals and a good insecticide/pesticide.
Specifications | MSDS | Literature and Reviews
2,2′:5′,2′′-Terthiophene (CAS number 1081-34-1), also known as α-terthienyl, can be considered as an oligothiophene and it has a structure of three thiophene units joined at α-positions of thiophene rings. α-terthienyl, a naturally occurring secondary plant metabolite, is found in abundance in the roots of marigold (family Asteraceae). It is toxic to several insect species while activated by ultraviolet light. It has capacity of inhibiting several enzymes, both in vivo and in vitro by generating oxygen radical species. α-terthienyl possesses all the desirable properties of a good insecticide/pesticide. It is fast acting, non-toxic, economic and its property of degradation makes it more user friendly and safe. Secondary plant metabolites will play an important role in future insecticide development programme.
2,2′:5′,2′′-Terthiophene can be prepared by reacting 2,5-dibromothiophene with Grignard reagent via Kumada, stannylthiophene via Stille, a thiophene boronic acid or boronate ester via Suzuki coupling reactions, or cyclization of 2-thienylacetylenes.
5,5″-dicyano-2,2′:5′,2″-terthiophene can be prepared from α-terthienyl. Polarizing optical microscopy shows that the oligothiophene derivatives have liquid crystalline properties. The crystalline phases of the oligothiophene derivatives showed molecular orientation.
General Information
| CAS Number | 1081-34-1 |
| Chemical Formula | C12H8S3 |
| Full Name | 2,2′:5′,2′′-terthiophene |
| Molecular Weight | 248.39 g/mol |
| Synonyms | α-terthienyl, 2,5-Di(2-thienyl)thiophene, ,2‘:5‘,2‘ ‘-Terthienyl |
| Classification / Family | Terthiophene, semiconductor synthesis intermediates, low band gap polymers, OLED, OFETs, organic photovoltaics |
Chemical Structure
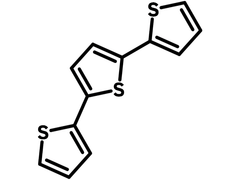
Product Details
| Purity | >98% (1H NMR in CDCl3) |
| Melting Point | 95.0 °C |
| Appearance | Off-white to orange-brown powder/crystals |
MSDS Documentation
 2,2′:5′,2′′-terthiophene MSDS Sheet
2,2′:5′,2′′-terthiophene MSDS Sheet
Literature and Reviews
- Alpha-terthienyl: A plant-derived new generation insecticide, M. Nivsarkar et al., Curr. Sci., 81(6), 667-672 (2001).
- Process Design and Scale-Up of the Synthesis of 2,2‘:5‘,2‘ ‘-Terthienyl, B. Smeets et al., Org. Proc. Res. Dev., 7 (1), 10–16 (2003); DOI: 10.1021/op020044n.
- Influence of the molecular orientation of oligothiophene derivatives in vacuum-evaporated thin films on photovoltaic properties, J. Hu et al., Dyes Pigm., 100, 158-161 (2014); DOI: 10.1016/j.dyepig.2013.09.010.
