IDTTB6-2Sn
CAS Number 1420071-65-3
Chemistry Building Blocks, Heterocyclic Building Blocks, Materials, Monomers, Organotin CompoundsHigh purity IDTTB6-2Sn available to buy online
Intermediate for the synthesis of non-fullerene acceptors or low bandgap semiconducting polymers
Specifications | MSDS | Literature and Reviews
6,6,12,12-tetrakis(4-hexylphenyl)-s-indacenodithieno[3,2-b]thiophene-bis(trimethylstannane) (IDTTB6-2Sn), or 6,6,12,12-tetrakis(4-hexylphenyl)-s-indacenodithieno[3,2-b]thiophene-bis(trimethylstannane), is an useful intermediate for the synthesis of either non-fullerene acceptors (NFAs) or low bandgap semiconducting polymers. Polymer L1057 nanoparticles, which are used for NIR-II bioimaging-guided photothermal cancer therapy, has IDTTB6-2Sn as one of its building blocks [2].
IDTTB6-2Sn has a fused indacenodithieno[3,2-b]thiophene (IDTT) back-bone with out-of-plane hexylphenyl side chains. Trimethylstannyl end functional groups provide access to C-C formation via Stille coupling reaction.
Part of our range of non-fullerene acceptor monomers, high purity (>98%) IDTTB6-2Sn is available for priority dispatch (lead times may apply for large quantities) and qualifying orders ship free.
Enabling facile coupling reaction
with trimethylstannyl end groups
High purity
98% IDTTB6-2Sn Purity
Worldwide shipping
Quick and reliable shipping
Electron-rich building block
For the preparation of low bandgap non-fullerene acceptors (NFAs)
General Information
| CAS Number | 1420071-65-3 |
| Chemical Formula | C74H90S4Sn2 |
| Molecular Weight | 1345.17 g/mol |
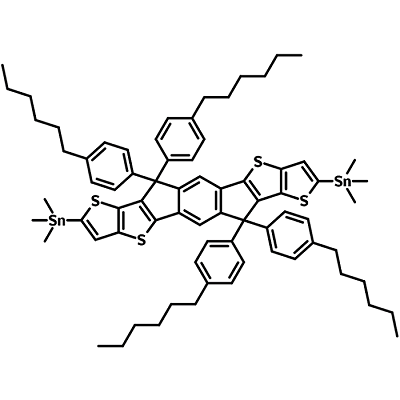
| Full Name | 6,6,12,12-tetrakis(4-hexylphenyl)-s-indacenodithieno[3,2-b]thiophene-bis(trimethylstannane) |
| Synonyms |
BT-IDT-nC6 3,9-bis(trimethylstannane)-5,5,11,11-tetrakis(4-hexylphenyl)-dithieno[2,3-d:2′,3′-d′]-s-indaceno[1,2-b:5,6-b′]-dithiophene 6,6,12,12-Tetrakis(4-hexylphenyl)-6,12-dihydrodithieno[2,3-d:2',3'-d']-s-indaceno[1,2-b:5,6-b']dithiophene-2,8-bis(trimethylstannane) |
| Classification / Family | Indacenodithieno[3,2-b]thiophene (IDTT), monomer and intermediates, non-fullerene acceptors (NFAs), NFA-OSCs, printing electronics |
Chemical Structure
Product Details
| Purity | >98% (by NMR) |
| Melting Point | N/A |
| Appearance | Yellow crystalline powder |
MSDS Documentation
Literature and Reviews
- Enhanced Performance of Organic Solar Cells with Increased End Group Dipole Moment in Indacenodithieno[3,2-b]thiophene-Based Molecules, J. Intemann et al., Adv. Funct. Mater., 25 (30), 4889-4897 (2015); DOI: 10.1002/adfm.201501600.
- Semiconducting Polymer Nanoparticles as Theranostic System for Near-Infrared-II Fluorescence Imaging and Photothermal Therapy under Safe Laser Fluence, Y. Yang et al., ACS Nano, 14, 2, 2509–2521 (2020); DOI: 10.1021/acsnano.0c00043.
- Effect of backbone structure on the thermoelectric performance of indacenodithiophene-based conjugated polymers, C. Wei et al., React. Funct. Polym., 142, 1-6 (2019); DOI: 10.1016/j.reactfunctpolym.2019.05.015.

 IDTTB6-2Sn MSDS Sheet
IDTTB6-2Sn MSDS Sheet