5,5′′-Dibromo-2,2′:5′,2′′-terthiophene
CAS Number 98057-08-0
Chemistry Building Blocks, Dibromo Monomers, Heterocyclic Building Blocks, Materials, Monomers5,5′′-Dibromo-2,2′:5′,2′′-terthiophene
This intermediate is widely used for the synthesis of semiconducting molecules, oligomers and conjugated polymers.
Specifications | MSDS | Literature and Reviews
5,5′′-Dibromo-2,2′:5′,2′′-terthiophene (CAS number 98057-08-0) is a brominated derivative of α-terthienyl at 5,5′′-positions. 5,5′′-Dibromo-2,2′:5′,2′′-terthiophene can be prepared via bromination with N-Bromosuccinimide in N,N-dimethylformamide (DMF).
5,5′′-Dibromo-2,2′:5′,2′′-terthiophene is a useful building block for the further synthesis of small molecules, oligomers and conjugated semiconducting polymers. Pyrene end-capped thiophene oligomer 5,5′′-di(pyren-1-yl)-2,2′:5′,2′′-terthiophene prepared from one step synthesis from 5,5′′-Dibromo-2,2′:5′,2′′-terthiophene show great potential as semiconducting materials for OTFTs and OSCs. Devices based on 5,5′′-di(pyren-1-yl)-2,2′:5′,2′′-terthiophene exhibited a field effect mobility of 0.11 cm2 V−1 s−1. Naphthyl end-capped oligothiophenes are also good candidates for high-performance organic electronic devices. Methoxy-functionalized 5,5′′-bis-(6-methoxynaphth-2-yl)-2:2′,5′:2′′-terthiophene (MONaT3) substantially increases the crystallization into aligned fibers.
General Information
| CAS Number | 98057-08-0 |
| Chemical Formula | C12H6Br2S3 |
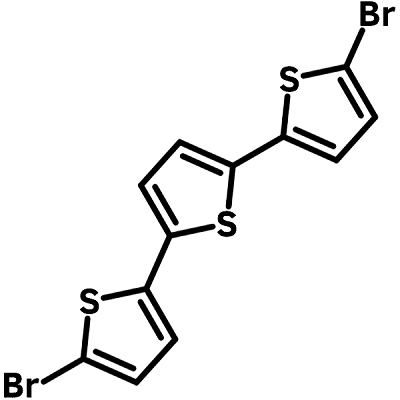
| Full Name | 5,5′′-Dibromo-2,2′:5′,2′′-terthiophene |
| Molecular Weight | 406.18 g/mol |
| Synonyms | 2,5-Bis(5-bromothiophen-2-yl)thiophene |
| Classification / Family | Terthiophene, semiconductor synthesis intermediates, low band gap polymers, OLED, OFETs, organic photovoltaics |
Chemical Structure
Product Details
| Purity | >98% (1H NMR in CDCl3) |
| Melting Point | 158.0 °C |
| Appearance | Light yellow powder/crystals |
MSDS Documentation
 5,5′′-Dibromo-2,2′:5′,2′′-terthiophene MSDS Sheet
5,5′′-Dibromo-2,2′:5′,2′′-terthiophene MSDS Sheet
Literature and Reviews
- Selective Synthesis of α-Substituted Oligothiophenes, P. Bauerle, et al., Synthesis,11, 1099-1103 (1993); DOI:10.1055/s-1993-26009.
- Novel Electron Acceptors Bearing a Heteroquinonoid System. I. Synthesis and Conductive Complexes of 5,5′-Bis(dicyanomethylene)-5,5′-dihydro-Δ2,2′-bithiophene and Related Compounds, Y. Koji et al., Bull. Chem. Soc. Jpn., 62 (5), 1539-1546 (1989); DOI: 10.1246/bcsj.62.1539.
- Synthesis and liquid crystal properties of a novel family of oligothiophene derivatives, P. Liu et al., Tetrahedron 60 (24), 5259-5264 (2004); DOI: 10.1016/j.tet.2004.04.029.
