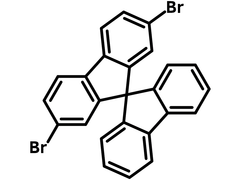
2,7-Dibromo-9,9′-spirobifluorene
CAS Number 171408-84-7
Chemistry Building Blocks, Dibromo Monomers, Materials, Monomers, Non-Heterocyclic Building BlocksHigh-Purity 2,7-Dibromo-9,9′-spirobifluorene
Intermediate used for the synthesis of semiconducting molecules, polymers with a spirobifluorene core for applications in highly efficient OLEDs and perovskite solar cells.
Specifications | MSDS | Literature and Reviews
2,7-Dibromo-9,9′-spirobifluorene (CAS number 171408-84-7) is a double brominated derivative of 9,9′-spirobifluorene, only brominated at one of the joined fluorenes.
Bromos at 2,7-positions give 2,7-dibromo-9,9′-spirobifluorene ability to obtain derivatives with varying electronic characteristics and physical properties. The spirobifluorene core at 9-positon can provide certain characteristics like higher triplet energy, elevated glass transition temperature, and thermal and chemical stabilities. It offers the prospect of controlling the molecular interaction of fluorene derivatives in the solid state by its sp3 carbon and twisted non-coplanar structure, preventing the close packing of molecules and generating amorphous glass materials with morphological stability. The connecting sp3 carbon also breaks the conjugation inside the molecule ending with a higher triplet energy. The twisted structure also gives more room for further modification at 2,7-positions.
2,7-Dibromo-9,9′-spirobifluorene can be prepared from 2,7-dribromo-9-fluorenone by reacting with a Grignard reagent of 2-bromobiphenyl. The most commonly known spirobifluorene based hole transport layer material is N2,N7-di(naphthalen-1-yl)-N2,N7-diphenyl-9,9′-spirobi[fluorene]-2,7-diamine (Spiro-NPB), and electron transport layer material 2,7-bis(diphenylphosphoryl)-9,9'-spirobifluorene (SPPO13, M2333A1).
Spirobifluorene building block
for the synthesis of OLED and organic photovoltaic materials
Worldwide shipping
Quick and reliable shipping
Capped with bromides
for facile coupling reactions
High purity
>99% Purity
General Information
| CAS Number | 171408-84-7 |
| Chemical Formula | C25H14Br2 |
| Full Name | 2,2′,7,7′-Dibromo-9,9′-spirobifluorene |
| Molecular Weight | 474.19 g/mol |
| Synonyms | 2,7-Dibromo-9,9′-spirobi[9H-fluorene], DBSBF |
| Classification / Family | Spirobifluorene, semiconductor synthesis intermediates, low band gap polymers, OLED, OFETs, organic photovoltaics |
Chemical Structure
Product Details
| Purity | >99% (HPLC) |
| Melting Point | Tm = 334 °C - 336 °C |
| Appearance | White to off-white powder/crystals |
MSDS Documentation
 2,7-Dibromo-9,9′-spirobifluorene MSDS sheet
2,7-Dibromo-9,9′-spirobifluorene MSDS sheet
Literature and Reviews
- Improved Synthesis of 2,2‘-Dibromo-9,9‘-spirobifluorene and Its 2,2‘-Bisdonor-7,7‘-bisacceptor-Substituted Fluorescent Derivatives, C. Chiang et al., Org. Lett. , 7, 17, 3717–3720 (2005); DOI: 10.1021/ol0513591.
- Efficient Greenish Blue Electrochemiluminescence from Fluorene and Spirobifluorene Derivatives, F. Polo et al., J. Am. Chem. Soc., 134, 37, 15402–15409 (2012); DOI: 10.1021/ja3054018.
- Red-Emitting Fluorenes as Efficient Emitting Hosts for Non-Doped, Organic Red-Light-Emitting Diodes, C. Chiang et al., Adv. Funct. Mater., 15 (2), 231-238 (2005); DOI: 10.1002/adfm.200400102.
