BP4mPy
CAS Number 1009033-94-6
High Purity Sublimed Materials, Semiconducting Molecules, TADF Materials
BP4mPy, one of the most popular ETL and HBL material used in OLEDs
Paired with electron-donating materials to host red, green, and blue PhOLEDs
BP4mPy, namely 3,3',5,5'-Tetra[(m-pyridyl)-phen-3-yl]biphenyl, is one of the most popular electron-transporting and hole-blocking layer materials used in OLEDs. It is electron deficient due to the electron-withdrawing nature of its four pyridine pendants. Together with electron-donating materials (e.g. TCTA), BP4mPy can also be used as an exciplex host for red, green, and blue phosphorescent OLEDs. BP4mPy aids effective energy transfer from exciplexes to emitters, thus leading to high efficiencies.
General Information
| CAS number | 1009033-94-6 |
|---|---|
| Full name | 3,3',5,5'-Tetra[(m-pyridyl)-phen-3-yl]biphenyl, 3,5,3',5'-Tetra(3-pyrid-3-ylphenyl)-1,1'-biphenyl. |
| Chemical formula | C56H38N4 |
| Molecular weight | 766.93 g/mol |
| Absorption | λmax 252 nm in THF |
| Fluorescence | λmax 352 nm in THF |
| HOMO/LUMO | HOMO = 6.66 eV, LUMO = 2.57 eV [1] |
| Classification / Family | Electron-transport layer (ETL) materials, Hole-blocking layer (HBL) materials, TADF materials. |
Product Details
| Purity | Sublimed > 99% (HPLC) |
|---|---|
| Melting point | Tg = 105 °C |
| Appearance | Off-white crystals/powder |
Chemical Structure
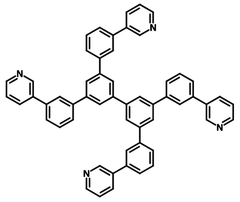
Device Structure(s)
| Device structure | ITO (90 nm)/TAPC (65 nm)/TCTA (5 nm)/26DCzPPy:4 wt% B-2PXZ (30 nm)/BP4mPy (40 nm)/LiF (0.8 nm)/Al (150 nm) [2] |
|---|---|
| Colour | Yellow |
| Max. Power Efficiency | 20.3 lm W−1 |
| Max. Current Efficiency | 32.2 cd/A |
| Max. EQE | 10.1% |
| Device structure | ITO (110 nm)/TAPC (30 nm)/mCP:1.0 wt% Os(bpftz)2(PPhMe2)2* (1 nm)/mCP:8.0 wt% Ir(bptz)2(bdp)* (18 nm)/mCP:7.0 wt% Os(bpftz)2(PPhMe2)2* (1 nm)/BP4mPy (50 nm)/LiF (0.8 nm)/Al (150 nm) [3] |
|---|---|
| Colour | White |
| Max. Power Efficiency | 10.39 lm W−1 |
| Max. Current Efficiency | 13.25 cd/A |
| Max. EQE | 6.16% |
| Device structure | ITO/TAPC (40 nm)/mCP:Complex 2* 1 wt% (30 nm)/BP4mPy (40 nm)/LiF (0.8 nm)/Al (150 nm) [4] |
|---|---|
| Colour | Green |
| Max. Power Efficiency | 22.6 lm W−1 |
| Max. Current Efficiency | 30.3 cd/A |
| Max. EQE | 10.0% |
| Device structure | ITO (90 nm)/TAPC:20 wt % of MoO3 (20 nm)/TAPC (30 nm)/26DCzPPy and x wt % PXZBM* (30 nm)/BP4mPy (40 nm)/LiF (0.8 nm)/Al (150 nm) [5] |
|---|---|
| Colour | Green |
| Max. Power Efficiency | 50.0 lm W−1 |
| Max. Current Efficiency | 67.7 cd/A |
| Max. EQE | 22.6% |
| Device structure | ITO (110 nm)/TAPC (30 nm)/mCP and 8.0 wt% [Os(pz2py)(PPh2Me)2(CO)]* (30 nm)/BP4mPy (50 nm)/LiF (0.8 nm)/Al (150 nm) [6] |
|---|---|
| Colour | Yellow |
| Max. Power Efficiency | 53.8 lm W−1 |
| Max. Current Efficiency | 61.0 cd/A |
| Max. EQE | 13.8% |
| Device structure | ITO/TAPC (40 nm)/TCTA 46 wt %:BP4mPy 46 wt %:fac-Ir(ppy)3 8 wt % (30 nm)/BP4mPy (40 nm)/LiF (0.8 nm)/Al (150 nm) [7] |
|---|---|
| Colour | Green |
| Max. Power Efficiency | 42.6 lm W−1 |
| Max. Current Efficiency | 48.7 cd/A |
| Max. EQE | 14.1% |
| Device structure | ITO/TAPC (40 nm)/TCTA 46 wt %:BP4mPy 46 wt %:FIrpic 8 wt % (30 nm)/BP4mPy (40 nm)/LiF (0.8 nm)/Al (150 nm) [7] |
|---|---|
| Colour | Blue |
| Max. Power Efficiency | 37.9 lm W−1 |
| Max. Current Efficiency | 35.6 cd/A |
| Max. EQE | 15.8% |
*For chemical structure information, please refer to the cited references
Pricing
| Grade | Order Code | Quantity | Price |
|---|---|---|---|
| Sublimed (>99% purity) | M2178A1 | 100 mg | £220 |
| Sublimed (>99% purity) | M2178A1 | 250 mg | £440 |
| Sublimed (>99% purity) | M2178A1 | 500 mg | £740 |
| Sublimed (>99% purity) | M2178A1 | 1 g | £1250 |
MSDS Documentation
Literature and Reviews
- Near infrared-emitting tris-bidentate Os(II) phosphors: control of excited state characteristics and fabrication of OLEDs, J. Liao et al., J. Mater. Chem. C, 3, 4910 (2015); DOI: 10.1039/c5tc00204d.
- Efficient donor-acceptor-donor borylated compounds with extremely small ΔEST for thermally activated delayed fluorescence OLEDs, C. Tsai et al., Org. Electron., 63, 166–174 (2018); DIO: 10.1016/j.orgel.2018.09.023.
- Blue-emitting Ir(III) phosphors with 2-pyridyl triazolate chromophores and fabrication of sky blue- and white emitting OLEDs, C. Chang et al., J. Mater. Chem. C, 1, 2639 (2013); DOI: 10.1039/c3tc00919j.
 BP4mPy MSDS sheet
BP4mPy MSDS sheet