4,4'-Dibromo-2,2'-dinitrobiphenyl
CAS Number 91371-12-9
Chemistry Building Blocks, Dibromo Monomers, Materials, Monomers, Non-Heterocyclic Building BlocksA useful readily available biphenyl intermediate
Used for the synthesis of carbazoles, dibenzosiloles and dibenzoselenophenes
Specifications | MSDS | Literature and Reviews
4,4'-Dibromo-2,2'-dinitrobiphenyl (CAS number 91371-12-9) is a biphenyl intermediate with strong electron withdrawing dinitro groups at 2,2'-positions and dibromo groups at 4,4'-positions. It is a well-known intermediate for the synthesis of 2,7-dibromo-9H-carbazole, shown below.
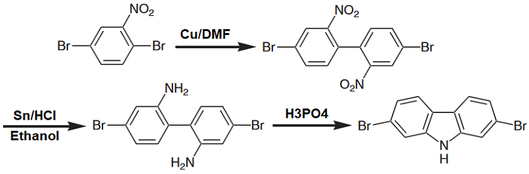
4,4'-Dibromo-2,2'-dinitrobiphenyl can be obtained from one step synthesis from 2,5-dibromonitrobenzene via Ullmann coupling reaction in dimethylformamide (DMF) in high yield. Further reduction of 4,4'-Dibromo-2,2'-dinitrobiphenyl affords 4,4’-dibromobiphenyl-2,2'-diamine which is another useful intermediate for the formation of fused heterocyclic rings such as carbazoles, dibenzosiloles and dibenzoselenophenes.
Derived from 4,4′-dibromo-2,2′-dinitrobiphenyl, 9-phenyl-9-phosphafluorene oxide (PhFlOP) based TADF emitters achieved outstanding electroluminescence performances with the maximum external quantum efficiency of 23.3%, current efficiency of 83.7 cd/A, and power efficiency of 59.1 lm W-1.
General Information
| CAS Number | 91371-12-9 |
| Chemical Formula | C12H6Br2N2O4 |
| Full Name | 4,4'-Dibromo-2,2'-dinitrobiphenyl |
| Molecular Weight | 402.00 g/mol |
| Synonyms | 4,4'-Dibromo-2,2'-dinitro-1,1'-biphenyl, 4-bromo-1-(4-bromo-2-nitrophenyl)-2-nitrobenzene |
| Classification / Family | Biphenyl derivatives, Semiconductor synthesis intermediates, OLED, OFETs, organic photovoltaics |
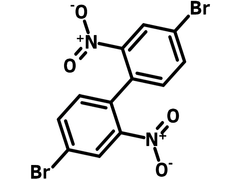
Chemical Structure
Product Details
| Purity | >98% (1H NMR) |
| Melting Point | Tm = 148 °C – 150 °C |
| Appearance | Light-yellow powder/crystals |
MSDS Documentation
 4,4'-Dibromo-2,2'-dinitrobiphenyl MSDS Sheet
4,4'-Dibromo-2,2'-dinitrobiphenyl MSDS Sheet
Literature and Reviews
-
Organic Emitters with a Rigid 9-Phenyl-9-phosphafluorene Oxide Moiety as the Acceptor and Their Thermally Activated Delayed Fluorescence Behavior, D. Zhong et al., ACS Appl. Mater. Interfaces, 11 (30), 27112–27124 (2019); DOI: 10.1021/acsami.9b05950.
- Synthesis and fluorescent properties of conjugated co-oligomers containing maleimide and carbazole units at the main chain, M. Nakamura et al., Polym. J., 46, 94–103 (2014): DOI: 10.1038/pj.2013.83.
- Investigations on the electrochemical properties of new conjugated polymers containing benzo[c]cinnoline and oxadiazole moieties, Polymer, 52 (26), 6011-6019 (2011); DOI: 10.1016/j.polymer.2011.10.059.
