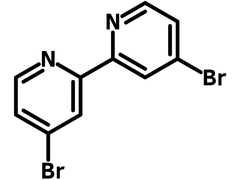
4,4'-Dibromo-2,2'-bipyridine
CAS Number 18511-71-2
Chemistry Building Blocks, Dibromo Monomers, Heterocyclic Building Blocks, Materials, MonomersA popular bipyridyl building blocks
Used for the synthesis of hole transport layers and photosensitizers for OLEDs and OPVs
Specifications | MSDS | Literature and Reviews
4,4'-Dibromo-2,2'-bipyridine (DBrBPy, CAS number 18511-71-2) is a 4,4'-brominated bipyridyl derivative joined at 2,2'-positions. 4,4'-Dibromo-2,2'-bipyridine is electron deficient and normally used as a ligand for the synthesis of organometallic compounds, i.e., ruthenium (II) photosensitizers.
Organic semiconductor with an electron withdrawing bipyridine core and triarylamine electron donating moieties can act as an efficient tunnel between perovskite and a hole transporting layer. It is believed that the bipyridine core can passivate the uncoordinated Pb2+ defects of perovskite films. As a result, the hole mobility of the hole transporting layer was increased by 7 times, from 1.05 × 10-4 to 8.36 × 10-4 cm2 V-1 s-1 and the power conversion efficiency (PCE) of n–i–p structured perovskite solar cells was boosted with improved stability [1].
2,2′-bipyridine bearing two corannulenes at 4.4-positions can act as molecular tweezers for fullerene recognition. The syn or anti confirmation can be selected simply by Cu(I) coordination/decoordination, thus managing the fullerene recognition capability of the system on demand [2].
General Information
| CAS Number | 18511-71-2 |
| Chemical Formula | C10H6Br2N2 |
| Full Name | 4,4'-Dibromo-2,2'-bipyridine |
| Molecular Weight | 313.98 g/mol |
| Synonyms | 4,4'-Dibromo-2,2'-bipyridyl, DBrBPy |
| Classification / Family | Bipyridine derivatives, Semiconductor synthesis intermediates, OLED, OFETs, organic photovoltaics |
Chemical Structure
Product Details
| Purity | >98% (1H NMR) |
| Melting Point | Tm = 138 °C |
| Appearance | White powder |
MSDS Documentation
 4,4'-Dibromo-2,2 -dipyridine MSDS Sheet
4,4'-Dibromo-2,2 -dipyridine MSDS Sheet
Literature and Reviews
-
A highly efficient interface hole transporting tunnel by a bipyridine semiconductor for perovskite solar cells, J. Mater. Chem. C, 10, 18069-18076 (2022); DOI: 10.1039/D2TC03649E.
-
ON/OFF metal-triggered molecular tweezers for fullerene recognition, A. Sacristán-Martín, Chem. Commun., 57, 11013 (2021); DOI: 10.1039/d1cc03451k.
-
Synthesis, properties, and OLED characteristics of 2,2′-bipyridine-based electron-transport materials: the synergistic effect of molecular shape anisotropy and a weak hydrogen-bonding network on molecular orientation, Y. Watanabe et al., J. Mater. Chem. C, 4, 3699-3704 (2016); DOI: 10.1039/C5TC03737A.
