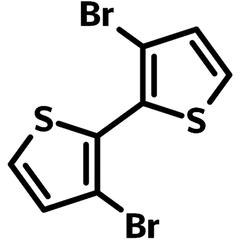
3,3'-Dibromo-2,2'-bithiophene
CAS Number 51751-44-1
Chemistry Building Blocks, Dibromo Monomers, Heterocyclic Building Blocks, Materials, Monomers3,3'-Dibromo-2,2'-bithiophene available to buy online
Key intermediate for the preparation of semiconducting molecules, oligothiophenes and polymers
Specifications | MSDS | Literature and Reviews
3,3'-Dibromo-2,2'-bithiophene (CAS number 51751-44-1) is an isomer to 5,5'-Dibromo-2,2'-bithiophene. It is a key intermediate for the preparation of semiconducting molecules, oligothiophenes and polymers which find applications in a variety of organic electronic and optoelectronic devices. These include organic field-effect transistors, chemical sensors, and organic solar cells.
Alkylation or arylation at 3-positions of the bithiophene not only provide either extended conjugation or improved solubility, but also give new functionality to change the electron and photophysical properties of the target materials. In addition, alkylation or arylation at 3-positions has a significant impact on the crystallinity of the target molecules to tune the morphology and the microstructure of the cast films.
Substitution of the bromines with either fluorine (F) or cyano (CN) groups makes the structure more electron deficient, which has a pounding effect on the device performance in terms of charge mobility, stability and overall OFET and OPV device efficiency.
3,3'-Dibromo-2,2'-bithiophene is also a must have building block for the synthesis of more complicated fused structures such as dithieno[3,2-b:2,3-d]pyrroles (DTP), dithieno[3,2-b:2,3-d]silole (DTS), dithieno[3,2-b:2,3-d]selenophene (DTSe), dithieno[3,2-b:2′,3′-d]germole (DTG) and 4,4′-Spiro-bis[cyclopenta[2,1-b;3,4-b′]dithiophene] (SCT).
Bithiophene building block
for the synthesis of OLED and organic photovoltaic materials
Worldwide shipping
Quick and reliable shipping
Capped with bromides
for facile coupling reactions
High purity
>98% Purity
General Information
| CAS Number | 51751-44-1 |
| Chemical Formula | C8H4Br2S2 |
| Full Name | 3,3'-Dibromo-2,2'-bithiophene |
| Molecular Weight | 324.06 g/mol |
| Synonyms | 2,2'-Bi(3-bromothiophene) |
| Classification / Family | Bithiophene, semiconductor synthesis intermediates, low band gap polymers, OLED, OFETs, organic photovoltaics |
Chemical Structure
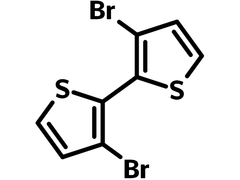
Product Details
| Purity | >98% (1H NMR in CDCl3) |
| Melting Point | 99 °C |
| Appearance | White-yellowish powder |
MSDS Documentation
 3,3'-Dibromo-2,2'-bithiophene MSDS Sheet
3,3'-Dibromo-2,2'-bithiophene MSDS Sheet
Literature and Reviews
- Simplified Synthetic Approach to Tetrabrominated Spiro-Cyclopentadithiophene and the Following Derivation to A-D-A Type Acceptor Molecules for Use in Polymer Solar Cells, X. Liu et al., J. Org. Chem., 87 (8), 5057–5064 (2022).
- Polymerization of Solid-State 2,2′-Bithiophene Thin Film or Doped in Cellulose Paper Using DBD Plasma and Its Applications in Paper-Based Electronics, K. Chen et al., ACS Appl. Polym. Mater., 2 (4), 1518–1527 (2020); DOI: 10.1021/acsapm.9b01202.
- Design of novel electroactive polybithiophene derivatives, K. Faid et al., Macromolecules, 26 (10), 2501–2507 (1993); DOI: 10.1021/ma00062a017.