1,8-Dibromonaphthalene
CAS Number 17135-74-9
Chemistry Building Blocks, Materials, Monomers, Non-Heterocyclic Building BlocksA well known double brominated naphthalene intermediate
leads to 1,8-diarylnaphthalenes in application of optoelectronic devices and chiral molecules studies.
Specifications | MSDS | Literature and Reviews
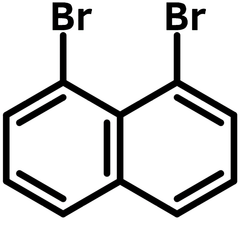
1,8-Dibromonaphthalene (1,8-DBN), CAS number 17135-74-9, is a symmetrical double bromo-substituted naphthalene with two bromos sitting at the naphthalene ring mirroring each other along the joining carbons. 1,8-Dibromonaphthalene is the building block for 1,8-diarylnaphthalenes, a fascinating class of strained organic molecules.
1,8-Diarylnaphthalenes are great example of strained organic molecules that have unusual parallel face-to-face arrangement of two peri-aryl rings in close proximity almost perpendicular to the central naphthalene backbone. 1,8-Diarylnaphthalenes show great potential in highly efficient photoluminescent blue and green OLEDs, chiral ligands and sensors, nonlinear optic chromophores, stereo-dynamic switches and other optoelectronic devices.
Naphthalene building block
For the synthesis of OLED and organic photovoltaic materials
Worldwide shipping
Quick and reliable shipping
Brominated at 1,8 positions
For strained organic molecules
High purity
>98% Purity
General Information
| CAS Number | 17135-74-9 |
| Chemical Formula | C10H6Br2 |
| Full Name | 1,8-Dibromonaphthalene |
| Molecular Weight | 285.97 g/mol |
| Synonyms | 1,8-DBN |
| Classification / Family | Naphthalenes, Semiconductor synthesis intermediates, Low band gap polymers, OLED, OFETs, organic photovoltaics |
Chemical Structure
Product Details
| Purity | >98% (1H NMR) |
| Melting Point | Tm = 110 °C |
| Appearance | White to Yellow, Pale Beige to Beige, Pale Brown to Brown powder/crystals |
MSDS Documentation
 1,8-Dibromonaphthalene MSDS Sheet
1,8-Dibromonaphthalene MSDS Sheet
Literature and Reviews
- Synthesis and Stereodynamics of Highly Constrained 1,8-Bis(2,2‘-dialkyl-4,4‘-diquinolyl)naphthalenes, G. Tumambac et al., J. Org. Chem., 69(6), 2048-2055 (2004); DOI: 10.1021/jo035547l.
- Atropisomerism of cofacial pyridine rings. Synthesis, proton NMR spectra and conformations of 1,8-di(3′-pyridyl)naphthalene, Tetrahedron, J. Zoltewicz et al., 52 (26), 8703-8706 (1996); DOI: 10.1016/0040-4020(96)00447-4.
- Naphthalene-1,8-diylbis(diphenylmethylium) as an Organic Two-Electron Oxidant: Benzidine Synthesis via Oxidative Self-Coupling of N,N-Dialkylanilines, T. Saitoh et al., J. Org. Chem., 71, 17, 6414–6419 (2006); DOI: 10.1021/jo060662s.
