1,5-Dibromonaphthalene
CAS Number 7351-74-8
Chemistry Building Blocks, Dibromo Monomers, Materials, Monomers, Non-Heterocyclic Building BlocksAn isomer of dibromonaphthalene and building block for organic semiconductors
Used in application of OLEDs and OFETs.
Specifications | MSDS | Literature and Reviews
1,5-Dibromonaphthalene (1,5-DBN), CAS number 7351-74-8, one of the many isomers of dibromonaphthalene, can be obtained by direct selective electrophilic substitution of 1-bromonaphthalene.
1,5-di(anthracen-2-yl)naphthalene (1,5-DAN), prepared from 1,5-dibromonaphthalene, has strong blue solid-state emission with high-efficiency charge transport ability. The single-crystal mobility of 1,5-DAN is as high as 8.41 cm2 V-1 s-1 with a photoluminescence quantum yield up to 20.54%. Unlike the 2,6-positions substituted molecules, 1,5-DAN displays a blue emission caused by the large torsion angle between the substituents and core [1].
Naphthalene building block
for the synthesis of OLED and organic photovoltaic materials
Worldwide shipping
Quick and reliable shipping
Brominated at 1,5 positions
for facile reactions
High purity
>98% Purity
General Information
| CAS Number | 7351-74-8 |
| Chemical Formula | C10H6Br2 |
| Full Name | 1,5-Dibromonaphthalene |
| Molecular Weight | 285.97 g/mol |
| Synonyms | 1,5-DBN |
| Classification / Family | Naphthalenes, Semiconductor synthesis intermediates, Low band gap polymers, OLED, OFETs, organic photovoltaics |
Chemical Structure
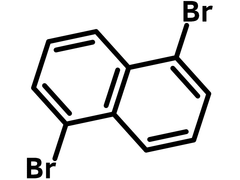
Product Details
| Purity | >98% (1H NMR) |
| Melting Point | Tm = 130 °C |
| Appearance | White to off-white powder/crystals |
MSDS Documentation
 1,5-Dibromonaphthalene MSDS Sheet
1,5-Dibromonaphthalene MSDS Sheet
Literature and Reviews
- Tailoring the substituted position for high-efficiency charge transport ability and strong blue solid-state emission in a naphthalene derivative, F. Li et al., Mater. Chem. Front., 5, 5124-5129 (2021); DOI: 10.1039/D1QM00452B.
- Synthesis of conjugated microporous polymer nanotubes for polymer composites, Z. Xiang et al., RSC Adv., 5, 24893-24898 (2015); DOI: 10.1039/C5RA00437C.
- Relationship between the Molecular Geometry and the Radiative Efficiency in Naphthyl-Based Bis-Ortho-Carboranyl Luminophores, S. Yi et al., Molecules, 27, 6565 (2022); DOI: 10.3390/molecules27196565.
