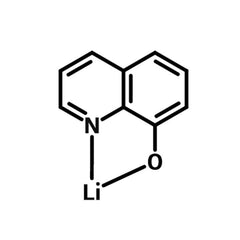
8-Quinolinolato lithium (Liq)
CAS Number 25387-93-3
Electron / Hole Transport Layer Materials, High Purity Sublimed Materials, Materials, Semiconducting Molecules
Liq, for efficient electron injection in organic electronic devices
To enhance the operational stability of LEDs
8-Hydroxyquinolinolato-lithium (Liq), coupled with aluminum (Al), is commonly used as an electron injection layer (EIL) material in organic electronic devices, particularly OLEDs. Normally, only a very thin layer (1-2 nm) Liq is needed for efficient electron injection from the electrode to the electron transport layer (ETL).
Liq/Al has also been widely known to be an effective cathode system towards general electron transport layer materials. It has also been reported that ultrathin Liq interlayers can greatly enhance the operational stability of light-emitting diodes [2].
General Information
| CAS number | 25387-93-3 |
|---|---|
| Chemical formula | C9H6LiNO |
| Molecular weight | 151.09 g/mol |
| Absorption | λmax 261 nm (in THF) |
| Fluorescence | λem 331 nm (in THF) |
| HOMO/LUMO | HOMO = 5.58 eV, LUMO = 3.15 eV [1] |
| Synonyms | Liq, Lithium-8-hydroxyquinolinolate, Lithium 8-quinolinolate, 8-Hydroxyquinolinolato lithium |
| Classification / Family | Electron transport layer (ETL) materials, Organic Light-Emitting Diodes, Organic electronics, Sublimed materials |
Product Details
| Purity | >99% (sublimed), >98% (unsublimed) |
|---|---|
| Melting point | 366-368 ºC (lit.) |
| TGA | Td ≥ 430 oC (5%) |
| DSC | Tm =365 oC (+/- 1oC) |
| Colour | Light yellow powder |
*Sublimation is a technique used to obtain ultra pure-grade chemicals. For more details about sublimation, please refer to the Sublimed Materials.
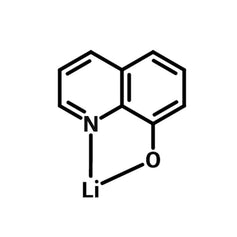
Chemical Structure
Device Structure(s)
| Device structure | ITO (150 nm)/NPB (70 nm)/mCP:Firpic-8.0%:Ir(ppy)3-0.5%:Ir(piq)3-0.5% (30 nm)/TPBi (30 nm)/Liq (2 nm)/Al (120 nm) [3] |
|---|---|
| Colour | White |
| Max. Luminance | 37,810 cd/m2 |
| Max. Current Efficiency | 48.1 cd/A |
| Device structure | ITO (180 nm)/TAPC (60 nm)/mCP:Firpic–8 wt% (10 nm)/Ir(ppz)3 (1.5 nm)/mCP:Firpic–8 wt% (10 nm)/Ir(ppz)3 (1.5 nm)/mCP:Firpic–8 wt% (10 nm)/TPBi (30 nm)/Liq (2 nm)/Al (120 nm) [4] |
|---|---|
| Colour | Blue |
| Luminance@200 cd/m2 | 32,570 cd/m2 |
| Max. Current Efficiency | 43.76 cd/A |
| Max. EQE | 23.4% |
| Max. Power Efficiency | 21.4 lm W−1 |
| Device structure | Al/MoO3 (3 nm)/mCP (50 nm)/Ir(tfmppy)2(tpip)* (0.5 nm)/TPBi (2.5 nm)/mCP (2.5 nm)/Ir(tfmppy)2(tpip) (0.5 nm)/TPBi (10 nm)/Bphen (45 nm)/Liq (1 nm)/Al (1 nm)/Ag (22 nm)/mCP (80 nm) [5] |
|---|---|
| Colour | Green |
| Max. Current Efficiency | 126.3 cd/A |
| Device structure | ITO/ NPB (70 nm)/DPVBi:BCzVBi (15 wt%, 15 nm)/ADN:BCzVBi (15% wt%, 15 nm)/BPhen (30 nm)/ Liq (2 nm)/Al (100 nm) [6] |
|---|---|
| Colour | Deep Blue |
| Max. Luminance | 8,668 cd/m2 |
| Max. Current Efficiency | 5.16 cd/A |
| Device structure | ITO/NPB/DPVBi:BCzVBi-6%/MADN:DCM2-0.5%/Bphen/Liq/Al [7] |
|---|---|
| Colour | White |
| Max. Luminance | 15,400 cd/m2 |
| Max. Current Efficiency | 6.19 cd/A |
| Device structure | ITO/PEDOT:PSS (40 nm)/ CzDMAC-DPS* (40 nm)/TPBI (40 nm)/Liq (1.6 nm)/Al (100 nm) [8] |
|---|---|
| Colour | Greenish-Blue |
| Max. Current Efficiency | 30.6 cd/A |
| Max. Power Efficiency | 12.2 lm W−1 |
| Device structure | ITO/HTL (100 nm)/CBP:9 wt%DACT-II*(40 nm)/BAlq (30 nm)/Liq/Al [9] |
|---|---|
| Colour | Green |
| Max. EQE | 41.3% |
*For chemical structure information, please refer to the cited references.
Characterisation

Pricing
| Grade | Order Code | Quantity | Price |
|---|---|---|---|
| Sublimed (>99% purity) | M731 | 1 g | £160 |
| Unsublimed (>98% purity) | M732 | 5 g | £150 |
| Sublimed (>99% purity) | M731 | 5 g | £520 |
MSDS Documentation
 8-Quinolinolato lithium MSDS sheet
8-Quinolinolato lithium MSDS sheet
Literature and Review
- Lithium-Quinolate Complexes as Emitter and Interface Materials in Organic Light-Emitting Diodes, C. Schmitz et al., Chem. Mater., 12, 3012-3019 (2000); doi: 10.1021/cm0010248
- Operational stability enhancement in organic light-emitting diodes with ultrathin Liq interlayers, DPK. Tsang et al., Sci Rep., 6: 22463 (2016); doi:10.1038/srep22463.
- Study of Sequential Dexter Energy Transfer in High Efficient Phosphorescent White Organic Light-Emitting Diodes with Single Emissive Layer, J-K. Kim et al., Sci. Reports, 4, 7009 (2014); DOI: 10.1038/srep07009.