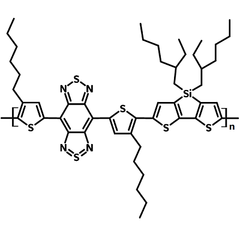
PBBTSiD (CAS number 1334032-16-4) is a small bandgap copolymer (Eg = ~ 0.7 eV ) with a backbone alternating electron donating dithienosilole (DTS) and electron accepting fused-ring benzobisthiadiazole (BBT) units.
The balanced ambipolarity of the BBT moiety of the polymer makes PBBTSiD a good candidate for logic circuit applications. Integrating BBT backboned semiconducting polymers into the bottom gate/top contact TFTs resulted in balanced ambipolar performances with the high µh and µe charge mobility values.
Luminosyn™ PBBTSiD
Luminosyn™ PBBTSiD is now available.High purity
PBBTSiD is purified via Soxhlet extraction with acetone, hexane, and chlorobenzene under an argon atmosphere
Large quantity orders
Plan your experiments with polymers from the same batch
General Information
| Full name | Poly[(4,7-bis(3-hexylthien-2-yl)- 2λ4δ2-benzo[1,2-c;4,5-c′]bis[1,2,5] thiadiazole)-alt-(3,3'-bis(2-ethylhexylsilyene-2,2'-bithiophene)] |
| Synonyms | PBBTSiD |
| Chemical formula | (C50H64N4S 6Si )n |
| CAS number | 1334032-16-4 |
| HOMO / LUMO | HOMO = -4.80 eV, LUMO = -4.10 eV; Eg = ~ 0.7 eV [1] |
| Solubility | Chloroform, chlorobenzene and dichlorobenzene |
| Processing solvent | Chlorobenzene and dichlorobenzene |
| Classification / Family | Organic semiconducting materials, Very low-bandgap polymers, Ambipolar semiconducting polymers, OFET polymers, High charge mobility polymers, Photodetectors, Thin-film Transistors |
Chemical Structure
UV-Vis-NIR Absorption
MSDS Documentation
Pricing
| Batch | Quantity | Price |
| M2258 A1 | 100 mg | £400 |
| M2258 A1 | 250 mg | £800 |
| M2258 A1 | 500 mg | £1450 |
| M2258 A1 | 1 g | £2600 |
| M2258 A1 | 5 g / 10 g* | Please contact us for details |
*for 5 - 10 grams order quantity, the lead time is 4-6 weeks.
Batch details
| Batch | Mw | Mn | PDI | Stock Info |
| M2258A1 | 17,975 | 7,554 |
2.38 | In stock |
Literature and Reviews
- Ambipolarity in Benzobisthiadiazole-Based Donor–Acceptor Conjugated Polymers, J. D. Yuen et al., Adv. Mater., 23, 3780–3785 (2011); DOI: 10.1002/adma.201101134.
- Organic Transistors in the New Decade: Toward n-Channel, Printed, and Stabilized Devices, S. Kola et al., J. Polym. Sci. B: Polym. Phys., 50, 1090–1120 (2012); DOI: 10.1002/polb.23054.
- Benzothiadiazole and its π-extended heteroannulated derivatives: useful acceptor building blocks for high-performance donor–acceptor polymers in organic electronics, Y. Wang et al., J. Mater. Chem. C, 4, 6200 (2016); DOI: 10.1039/c6tc01860b.
- Toward Printed Integrated Circuits based on Unipolar or Ambipolar Polymer Semiconductors, K. Baeg et al., Adv. Mater., 25 (31), 4210-4244 (2013); DOI: 10.1002/adma.201205361.


 PBBTSiD MSDS sheet
PBBTSiD MSDS sheet