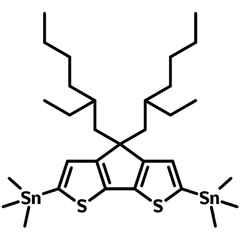
CDT-EH-Sn
CAS Number 920504-00-3
Chemistry Building Blocks, Heterocyclic Building Blocks, Monomers, Organotin Compounds
CDT-EH-Sn, namely 4,4-Bis(2-ethylhexyl)-2,6-bis(trimethylstannyl)-4H-cyclopenta[2,1-b:3,4-b']dithiophene, is the intermediate for the synthesis of low bandgap semiconducting polymers which are widely used in OLED, OPV and OFET devices.
4H-Cyclopenta[1,2-b:5,4-b']dithiophene (CDT) has electron rich bridged bithiophenes with a five member cyclopentadiene fused ring at its central core.
General Information
| CAS Number | 920504-00-3 |
| Chemical Formula | C31H54S2Sn2 |
| Molecular Weight | 728.3 g/mol |
| Full Name | 4,4-Bis(2-ethylhexyl)-2,6-bis(trimethylstannyl)-4H-cyclopenta[2,1-b:3,4-b']dithiophene |
| Synonyms | CDT26-2Sn 2,6-Bis(trimethyltin)-4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b']dithiophene |
| Classification / Family | cyclopenta[2,1-b:3,4-b']dithiophene (CDT) derivatives, Organic semiconducting materials, Semiconductor Synthesis, Low band gap polymers, Organic Photovoltaics. |
Chemical Structure
![CDT-EH-Sn, 2,6-bis(trimethyltin)-4,4-bis(2-ethylhexyl)-4h-cyclopenta[2,1-b:3,4-b']dithiophene, CAS 920504-00-3](https://cdn.shopify.com/s/files/1/0823/0287/files/CDT-EH-Sn-body_240x170.png?v=1613397450)
Product Details
| Purity | 97% |
| Boiling Point | N/A |
| Appearance | Yellowish liquid |
MSDS Documentation
Literature and Reviews
- Photoconductivity of a Low-Bandgap Conjugated Polymer, C. Soci et al., Adv. Funct. Mater., 17, 632–636 (2007); DOI: 10.1002/adfm.200600199.
- Synthesis and Characterization of Bridged Bithiophene-Based Conjugated Polymers for Photovoltaic Applications: Acceptor Strength and Ternary Blends, C-H. Chen et al., Macromolecules, 43, 697–708 (2010); DOI: 10.1021/ma902206u.
- Panchromatic Conjugated Polymers Containing Alternating Donor/Acceptor Units for Photovoltaic Applications, Z. Zhu et al., Macromolecules, 40, 6, 1981–1986 (2007); DOI: 10.1021/ma062376o..
Related Products
We stock a wide range of monomers available to purchase online. Please contact us if you cannot find what you are looking for.
 CDT-EH-Sn MSDS sheet
CDT-EH-Sn MSDS sheet
![2-Ethylhexyl 4,6-dibromo-3-fluorothieno[3,4-b]thiophene-2-carboxylate](http://www.ossila.com/cdn/shop/products/ptb7-monomer-b361-ossila-chemical-structure.png?v=1648818400&width=190)
![2,5-bis(trimethylstannyl)-thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/2_5-bis-trimethylstannyl-thieno-3_2-b-thiophene_structure.jpg?v=1504193831&width=190)
![2,6-dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/4Hcyclopentadithiophene.jpg?v=1431610575&width=190)
![2,7-Dibromo-9,9-bis[3,3'-(N,N-dimethylamino)-propyl]fluorene](http://www.ossila.com/cdn/shop/products/dibromo-fluorene-diyl-bisdimethylpropan-amine.jpg?v=1431610994&width=190)
![3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione](http://www.ossila.com/cdn/shop/products/bisbromothiophenyl-bisoctyldodecylpyrrolo-dione.jpg?v=1431611190&width=190)
![4H-Cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/cyclopentadithiophene_12a14774-f96a-4ebc-a0d0-2c5483da9180.jpg?v=1445441165&width=190)
![Benzo[1,2-b:4,5-b']dithiophene-4,8-dione](http://www.ossila.com/cdn/shop/products/benzo-dithiophene-dione.jpg?v=1437904702&width=190)
![Thienothiophene, Thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/thienothiophene.jpg?v=1431611114&width=190)
![Thieno[3,2-b]thiophene-2-carbonitrile](http://www.ossila.com/cdn/shop/products/thienothiophene-2-carbonitrile.jpg?v=1439548051&width=190)
![DTT, Dithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/dtt-chemical-structure.png?v=1653477307&width=190)
![3,6-Dibromothieno[3,2-b]thiophene (TT36)](http://www.ossila.com/cdn/shop/products/3-6-dibromothienothiophene-chemical-structure.png?v=1653663075&width=190)
![2,3,5,6-Tetrabromothieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/Tetrabromo-thienothiophene-chemical-structure.png?v=1665673773&width=190)
![2,6-Dibromodithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/Dibromodithienothiophene-chemical-structure.png?v=1666702461&width=190)
![2,5-Dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c]pyrrole-1,4-dione](http://www.ossila.com/cdn/shop/products/2_5-Dihydro-3_6-di-2-thienyl-pyrrolo_3_4-c_pyrrole-1_4-dione-chemical-structure-dpp.png?v=1667321819&width=190)
![6,9-bis(5-bromo-4-(2-butyloctyl)thiophen-2-yl)dithieno[3,2-f:2',3'-h]quinoxaline](http://www.ossila.com/cdn/shop/products/bisbromo-butyloctylthiophenyl-dithienoquinoxaline-chemical-structure.png?v=1669202898&width=190)
![Indolo[3,2-b]carbazole](http://www.ossila.com/cdn/shop/products/Indolocarbazole-chemical-structure.png?v=1670495077&width=190)
![10,15-Dihydro-5H-diindolo[3,2-a:3',2'-c]carbazole](http://www.ossila.com/cdn/shop/products/Dihydro-diindolocarbazole-chemical-structure.png?v=1670502109&width=190)
![Indolo[2,3-a]carbazole](http://www.ossila.com/cdn/shop/products/indolo-2-3-a-carbazole-chemical-structure-title.png?v=1678288567&width=190)
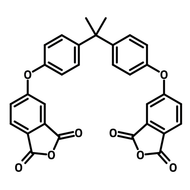
![2,2-Bis[4-(4-aminophenoxy)phenyl]propane (BAPP)](http://www.ossila.com/cdn/shop/products/bapp-chemical-structure-title.png?v=1679403349&width=190)
![2,2'-Dimethyl[1,1'-biphenyl]-4,4'-diamine](http://www.ossila.com/cdn/shop/products/2-2-dimethyl1-1-biphenyl-4-4-diamine-chemical-structure-title.png?v=1680597662&width=190)
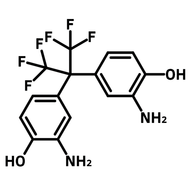
![2,2-Bis[4-(4-aminophenoxy)phenyl]hexafluoropropane (4-BDAF)](http://www.ossila.com/cdn/shop/products/4-bdaf-chemical-structure-title.png?v=1681225583&width=190)
![1-[2-(Trifluoromethyl)phenyl]imidazole](http://www.ossila.com/cdn/shop/files/1-2-trifluoromethylphenylimidazole-chemical-structure-title.png?v=1682593257&width=190)