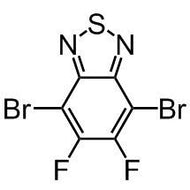
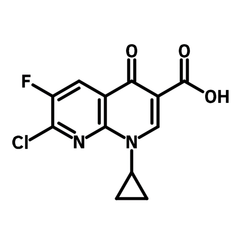
7-Chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid
CAS Number 100361-18-0
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Monomers
A fluorinated heterocyclic building block
Used as a synthesis intermediate for APIs in application of drug discovery
7-Chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid (CAS number 100361-18-0) is derived from naphthyridine, a bicyclic heterocyclic, bearing a chlorine (7-position), a fluorine (6-position), a cyclopropyl (1-position), a carbonyl (4-position) and a carboxylic acid (3-position). 7-Chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid is an important intermediate for synthesizing Gemifloxacin, a quinolone antibiotic. It is also a molecular scaffold for many other active pharmaceutical ingredients (APIs) of anticancer and anti-inflammatory activities.

7-Chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid has multiple metal-binding sides. The ketone and carboxylic acid form a 1,3-diketone moiety which binds transition metals to produce metal complexes. The drug-metal complexes show enhanced biological activity. The amines (1,8-position) in 7-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid can also chelate to metal centres yielding complexes for APIs.
Multiple functional groups
For facile synthesis
Fluorinated naphthyridine building block
For drug discovery, medicinal chemistry, and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 100361-18-0 |
| Chemical Formula | C12H18ClFN2O3 |
| Full Name | 7-Chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid |
| Molecular Weight | 282.65 g/mol |
| Synonyms | 1-Cyclopropyl-6-fluoro-7-chloride-4-oxo-1,4-dihydro-1,8-napthyridine-3-carboxylic acid |
| Classification / Family | Fluorinated building block, Heterocyclic building block, APIs, Antibiotics |
Chemical Structure

Product Details
| Purity | >98% |
| Melting Point | Tm = 224 °C |
| Appearance | Off-white powder |
MSDS Documentation
 7-Chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid MSDS Sheet
7-Chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid MSDS Sheet
Literature and Reviews
- 1,8-Naphthyridine derivatives: a review of multiple biological activities, A. Madaan et al., Arch. Pharm. Chem. Life Sci., 348, 837–860(2015); DOI: DOI 10.1002/ardp.201500237.
- 1,8-Naphthyridine revisited: applications dimetal chemistry, J. Bera et al., Eur. J. Inorg. Chem. 2009(27), 4023–4038(2009); DOI: 10.1002/ejic.200900312.
- 1,8-Naphthyridine-3-carboxamide derivatives with anticancer and anti-inflammatory activity, V. Kumar et al., Eur. J. Med. Chem., 44(8), 3356–3362(2009); DOI: 10.1016/j.ejmech.2009.03.015.
- Photophysics and photochemistry of nalidixic acid, P. Pavez et al., J. Photochem. Photobiol., 82, 254–261(2006); DOI: 10.1562/2005-04-11-RA-488.
- Quinolones: a comprehensive review, C. Oliphant et al., Am. Fam. Physician, 65(3), 455–464(2002); PMID: 11858629.
- Synthesis, characterization, antibacterial and anti-inflammatory activities of enoxacin metal complexes, S. Arayne et al., Bioinorg. Chem. Appl., 2009, 914105(2009); DOI: 10.1155/2009/914105.
Related Products
We stock a wide range of monomers available to purchase online. Please contact us if you cannot find what you are looking for.
![1-[2-(Trifluoromethyl)phenyl]imidazole](http://www.ossila.com/cdn/shop/files/1-2-trifluoromethylphenylimidazole-chemical-structure-title.png?v=1682593257&width=190)
![2,2-Bis[4-(4-aminophenoxy)phenyl]hexafluoropropane (4-BDAF)](http://www.ossila.com/cdn/shop/products/4-bdaf-chemical-structure-title.png?v=1681225583&width=190)
![2-Ethylhexyl 4,6-dibromo-3-fluorothieno[3,4-b]thiophene-2-carboxylate](http://www.ossila.com/cdn/shop/products/ptb7-monomer-b361-ossila-chemical-structure.png?v=1648818400&width=190)