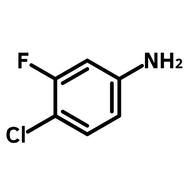
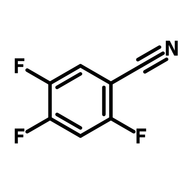
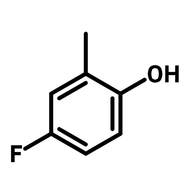
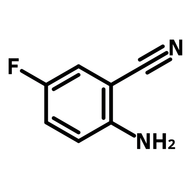
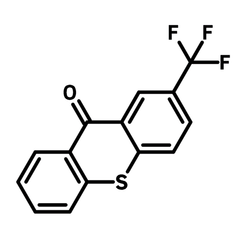
2-(Trifluoromethyl)thioxanthen-9-one
CAS Number 1693-28-3
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Monomers
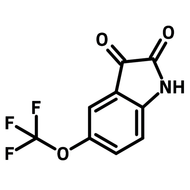
A fluorinated heterocyclic building block
Used as a synthesis intermediate for photosensitizers in application of photoredox catalytic reactions and OLEDs
2-(Trifluoromethyl)thioxanthen-9-one (CAS number 1693-28-3) is a trifluoromethyl substituted thioxanthenone/thioxanthone, a sulphur analogue of xanthone. The ketone on 2-(trifluoromethyl)thioxanthen-9-one is prone to nucleophilic reactions for molecular functionalization. Axially chiral thioxanthenes can be synthesized with this method (with organolithium compounds), exhibiting circularly polarized luminescence (CPL) with dissymmetry factor of 3.0×10−3 and maximum external quantum efficiency of 20.0% in circularly polarized OLEDs. The sulfide group can be oxidised to sulfone with oxidizing agent such as hydrogen peroxide and meta-chloroperoxybenzoic acid, for tuning the energy band gap of the molecule.
2-(Trifluoromethyl)thioxanthen-9-one is also desired of use as a triplet photosensitizer in photoredox catalytic reactions such as cycloaddition and nickel-catalyzed aryl esterification.
Multiple functional groups
For facile synthesis
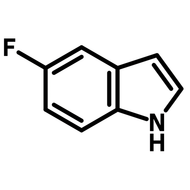
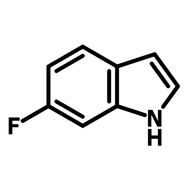
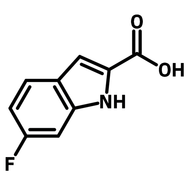
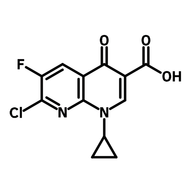
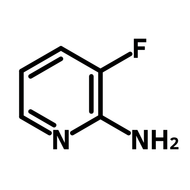
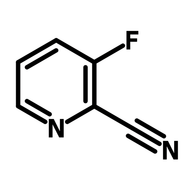
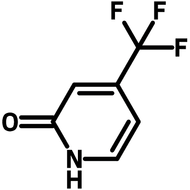
Fluorinated pyridone building block
For drug discovery, photocatalysts, and OLEDs
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 1693-28-3 |
| Chemical Formula | C14H7F3O3S |
| Full Name | 2-(Trifluoromethyl)-9H-thioxanthen-9-one |
| Molecular Weight | 280.26 g/mol |
| Synonyms | 2-Trifluoromethylthioxanthone |
| Classification / Family | Fluorinated building blocks, Heterocyclic building blocks, Photosensitizers, OLEDs, Photoredox reactions, Photocatalysts |
Chemical Structure

Product Details
| Purity | 98% |
| Melting Point | Tm = 147 °C – 151 °C |
| Appearance | White powder |
MSDS Documentation
 2-(Trifluoromethyl)thioxanthen-9-one MSDS Sheet
2-(Trifluoromethyl)thioxanthen-9-one MSDS Sheet
Literature and Reviews
-
Arene activation through iminium ions: product diversity from intramolecular photocycloaddition reactions, J. Proessdorf et al., Angew. Chem. Int. Ed., 61, e202208329(2022); DOI: 10.1002/anie.202208329.
-
Novel thermally activated delayed fluorescence materials-thioxanthione derivatives and their applications for highly efficient OLEDs, H. Wang et al., Adv. Mater., 26, 5198–5204(2014); DOI: 10.1002/adma.201401393.
-
Chiral spiro-axis induced blue thermally activated delayed fluorescence material for efficient circularly polarized OLEDs with low efficiency roll-off, Y.-P. Zhang et al., Angew. Chem. Int. Ed., 60(15), 8435–8440(2021); DOI: 10.1002/anie.202015411.
-
Visible light driven, nickel-catalyzed aryl esterification using a triplet photosensitizer thioxanthen-9-one, D.-L. Zhu et al., Org. Chem. Front., 6, 2353-2359(2019); DOI: 10.1039/C9QO00536F.
-
Tailoring valence tautomerism by using redox potentials: studies on ferrocene-based triarylmethylium dyes with electron-poor fluorenylium and thioxanthylium acceptors, L. Casper et al., Chem. Eur. J., 27, 10854–10868(2021); DOI: 10.1002/chem.202101032.
Related Products
We stock a wide range of monomers available to purchase online. Please contact us if you cannot find what you are looking for.
![2-Ethylhexyl 4,6-dibromo-3-fluorothieno[3,4-b]thiophene-2-carboxylate](http://www.ossila.com/cdn/shop/products/ptb7-monomer-b361-ossila-chemical-structure.png?v=1648818400&width=190)
![2,5-bis(trimethylstannyl)-thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/2_5-bis-trimethylstannyl-thieno-3_2-b-thiophene_structure.jpg?v=1504193831&width=190)
![2,6-dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/4Hcyclopentadithiophene.jpg?v=1431610575&width=190)
![2,7-Dibromo-9,9-bis[3,3'-(N,N-dimethylamino)-propyl]fluorene](http://www.ossila.com/cdn/shop/products/dibromo-fluorene-diyl-bisdimethylpropan-amine.jpg?v=1431610994&width=190)
![3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione](http://www.ossila.com/cdn/shop/products/bisbromothiophenyl-bisoctyldodecylpyrrolo-dione.jpg?v=1431611190&width=190)
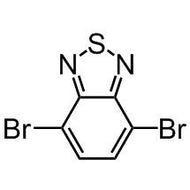
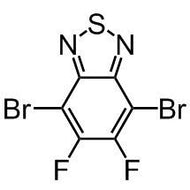
![4H-Cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/cyclopentadithiophene_12a14774-f96a-4ebc-a0d0-2c5483da9180.jpg?v=1445441165&width=190)
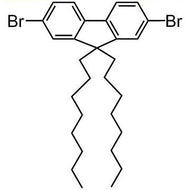
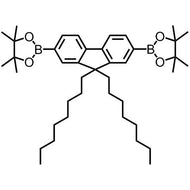
![Benzo[1,2-b:4,5-b']dithiophene-4,8-dione](http://www.ossila.com/cdn/shop/products/benzo-dithiophene-dione.jpg?v=1437904702&width=190)
![Thienothiophene, Thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/thienothiophene.jpg?v=1431611114&width=190)
![Thieno[3,2-b]thiophene-2-carbonitrile](http://www.ossila.com/cdn/shop/products/thienothiophene-2-carbonitrile.jpg?v=1439548051&width=190)
![DTT, Dithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/dtt-chemical-structure.png?v=1653477307&width=190)
![3,6-Dibromothieno[3,2-b]thiophene (TT36)](http://www.ossila.com/cdn/shop/products/3-6-dibromothienothiophene-chemical-structure.png?v=1653663075&width=190)
![2,3,5,6-Tetrabromothieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/Tetrabromo-thienothiophene-chemical-structure.png?v=1665673773&width=190)
![2,6-Dibromodithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/Dibromodithienothiophene-chemical-structure.png?v=1666702461&width=190)
![2,5-Dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c]pyrrole-1,4-dione](http://www.ossila.com/cdn/shop/products/2_5-Dihydro-3_6-di-2-thienyl-pyrrolo_3_4-c_pyrrole-1_4-dione-chemical-structure-dpp.png?v=1667321819&width=190)
![6,9-bis(5-bromo-4-(2-butyloctyl)thiophen-2-yl)dithieno[3,2-f:2',3'-h]quinoxaline](http://www.ossila.com/cdn/shop/products/bisbromo-butyloctylthiophenyl-dithienoquinoxaline-chemical-structure.png?v=1669202898&width=190)
![Indolo[3,2-b]carbazole](http://www.ossila.com/cdn/shop/products/Indolocarbazole-chemical-structure.png?v=1670495077&width=190)
![10,15-Dihydro-5H-diindolo[3,2-a:3',2'-c]carbazole](http://www.ossila.com/cdn/shop/products/Dihydro-diindolocarbazole-chemical-structure.png?v=1670502109&width=190)
![Indolo[2,3-a]carbazole](http://www.ossila.com/cdn/shop/products/indolo-2-3-a-carbazole-chemical-structure-title.png?v=1678288567&width=190)
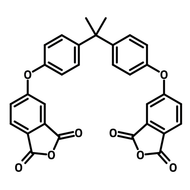
![2,2-Bis[4-(4-aminophenoxy)phenyl]propane (BAPP)](http://www.ossila.com/cdn/shop/products/bapp-chemical-structure-title.png?v=1679403349&width=190)
![2,2'-Dimethyl[1,1'-biphenyl]-4,4'-diamine](http://www.ossila.com/cdn/shop/products/2-2-dimethyl1-1-biphenyl-4-4-diamine-chemical-structure-title.png?v=1680597662&width=190)
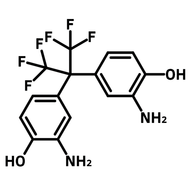
![2,2-Bis[4-(4-aminophenoxy)phenyl]hexafluoropropane (4-BDAF)](http://www.ossila.com/cdn/shop/products/4-bdaf-chemical-structure-title.png?v=1681225583&width=190)
![1-[2-(Trifluoromethyl)phenyl]imidazole](http://www.ossila.com/cdn/shop/files/1-2-trifluoromethylphenylimidazole-chemical-structure-title.png?v=1682593257&width=190)