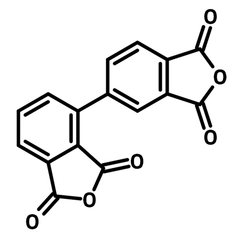
2,3,3',4'-Biphenyltetracarboxylic dianhydride (a-BPDA)
CAS Number 36978-41-3
Chemistry Building Blocks, Diamines and Dianhydrides, Heterocyclic Building Blocks, Monomers
A non-symmetrical biphenyl dianhydride
For the synthesis of polyimides in application of batteries, electrochromics, and high performance polymers
2,3,3',4'-Biphenyltetracarboxylic dianhydride (a-BPDA, CAS number 36978-41-3), a derivative of biphenyl, has two phthalic anhydrides linked unsymmetrically at 3- and 4-position. The backbone of the polyimides synthesised from 2,3,3',4'-Biphenyltetracarboxylic dianhydride is twisted, leading to a suppression of intermolecular charge transfer. Thus, the polyimides are transparent and heat resistant. Diimides synthesized from a-BPDA and cyclohexylamine display long-lived luminescence up to 1.3 seconds after ultraviolet light irradiation.
2,3,3',4'-Biphenyltetracarboxylic dianhydride is also used to synthesize covalent organic frameworks(COFs) with low dielectric constant (2.72), low thermal expansion coefficient (62.2 ppm/K) and high breakdown strength (412.8 kV/mm). It is ideal for low signal loss electronic packaging. Lithium battery cathodes of the polyimides-decorated carbon nanotubes (CNTs) show a high capacity of 163 mAh/g at 0.05 A/g and a rate capability of 122 mAh/g at 5 A/g.
General Information
| CAS Number | 36978-41-3 |
| Chemical Formula | C16H6O6 |
| Full Name | 2,3,3',4'-Biphenyltetracarboxylic dianhydride |
| Molecular Weight | 294.22 g/mol |
| Synonyms | 3,4'-Biphthalic anhydride, 4,5'-Bi-2-benzofuran-1,1',3,3'-tetrone |
| Classification / Family | Dianhydride building block, Polyimides, COFs, Batteries, High performance polymers, Electrochromics |
Chemical Structure

Product Details
| Purity | >99% |
| Melting Point | Tm = 195 °C – 205 °C |
| Appearance | White to off-white powder/crystal |
MSDS Documentation
 2,3,3',4'-Biphenyltetracarboxylic dianhydride (a-BPDA) MSDS Sheet
2,3,3',4'-Biphenyltetracarboxylic dianhydride (a-BPDA) MSDS Sheet
Literature and Reviews
-
Long-lived luminescence emitted from imide compounds dispersed in polymer matrices after continuous ultraviolet irradiation and its relation to oxygen quenching, M. Doi et al., ChemPhotoChem., e202200310(2023); DOI: 10.1002/cptc.202200310.
-
Colorless, heat resistant polyimide films derived from 2,3,3′,4′-biphenyltetracarboxylic dianhydride, G. Song et al., IOP Conf. Series: Materials Science and Engineering, 733, 012035(2020); DOI: 10.1088/1757-899X/733/1/012035.
-
Construction of all-organic low dielectric polyimide hybrids via synergistic effect between covalent organic framework and cross-linking structure, W. Zhao et al., Nano Mater. Sci., in press(2023); DOI: 10.1016/j.nanoms.2023.02.002.
-
Macroporous polyimide aerogels: a comparison between powder microparticles synthesized via wet gel grinding and emulsion process, S. Dayarian et al., Langmuir, 39, 1804-1814(2023); DOI: 10.1021/acs.langmuir.2c02696.
-
Novel polyimides containing flexible carbazole blocks with electrochromic and electrofluorescencechromic properties, R. Zheng et al., RSC Adv., 10, 6992(2020); DOI: 10.1039/c9ra10515h.
- Rational integration of carbon nanotubes into chain-engineered bipolar polyimides as core-shell heterostructured electrodes for polymer-based symmetrical full batteries, Q. Zhang et al., Adv. Funct. Mater., 33(5), 2211590(2022), DOI: 10.1002/adfm.202211590.
Related Products
We stock a wide range of monomers available to purchase online. Please contact us if you cannot find what you are looking for.
![2-Ethylhexyl 4,6-dibromo-3-fluorothieno[3,4-b]thiophene-2-carboxylate](http://www.ossila.com/cdn/shop/products/ptb7-monomer-b361-ossila-chemical-structure.png?v=1648818400&width=190)
![2,5-bis(trimethylstannyl)-thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/2_5-bis-trimethylstannyl-thieno-3_2-b-thiophene_structure.jpg?v=1504193831&width=190)
![2,6-dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/4Hcyclopentadithiophene.jpg?v=1431610575&width=190)
![2,7-Dibromo-9,9-bis[3,3'-(N,N-dimethylamino)-propyl]fluorene](http://www.ossila.com/cdn/shop/products/dibromo-fluorene-diyl-bisdimethylpropan-amine.jpg?v=1431610994&width=190)
![3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione](http://www.ossila.com/cdn/shop/products/bisbromothiophenyl-bisoctyldodecylpyrrolo-dione.jpg?v=1431611190&width=190)
![4H-Cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/cyclopentadithiophene_12a14774-f96a-4ebc-a0d0-2c5483da9180.jpg?v=1445441165&width=190)
![Benzo[1,2-b:4,5-b']dithiophene-4,8-dione](http://www.ossila.com/cdn/shop/products/benzo-dithiophene-dione.jpg?v=1437904702&width=190)
![Thienothiophene, Thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/thienothiophene.jpg?v=1431611114&width=190)
![Thieno[3,2-b]thiophene-2-carbonitrile](http://www.ossila.com/cdn/shop/products/thienothiophene-2-carbonitrile.jpg?v=1439548051&width=190)
![DTT, Dithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/dtt-chemical-structure.png?v=1653477307&width=190)
![3,6-Dibromothieno[3,2-b]thiophene (TT36)](http://www.ossila.com/cdn/shop/products/3-6-dibromothienothiophene-chemical-structure.png?v=1653663075&width=190)
![2,3,5,6-Tetrabromothieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/Tetrabromo-thienothiophene-chemical-structure.png?v=1665673773&width=190)
![2,6-Dibromodithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/Dibromodithienothiophene-chemical-structure.png?v=1666702461&width=190)
![2,5-Dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c]pyrrole-1,4-dione](http://www.ossila.com/cdn/shop/products/2_5-Dihydro-3_6-di-2-thienyl-pyrrolo_3_4-c_pyrrole-1_4-dione-chemical-structure-dpp.png?v=1667321819&width=190)
![6,9-bis(5-bromo-4-(2-butyloctyl)thiophen-2-yl)dithieno[3,2-f:2',3'-h]quinoxaline](http://www.ossila.com/cdn/shop/products/bisbromo-butyloctylthiophenyl-dithienoquinoxaline-chemical-structure.png?v=1669202898&width=190)
![Indolo[3,2-b]carbazole](http://www.ossila.com/cdn/shop/products/Indolocarbazole-chemical-structure.png?v=1670495077&width=190)
![10,15-Dihydro-5H-diindolo[3,2-a:3',2'-c]carbazole](http://www.ossila.com/cdn/shop/products/Dihydro-diindolocarbazole-chemical-structure.png?v=1670502109&width=190)
![Indolo[2,3-a]carbazole](http://www.ossila.com/cdn/shop/products/indolo-2-3-a-carbazole-chemical-structure-title.png?v=1678288567&width=190)
![2,2-Bis[4-(4-aminophenoxy)phenyl]propane (BAPP)](http://www.ossila.com/cdn/shop/products/bapp-chemical-structure-title.png?v=1679403349&width=190)
![2,2'-Dimethyl[1,1'-biphenyl]-4,4'-diamine](http://www.ossila.com/cdn/shop/products/2-2-dimethyl1-1-biphenyl-4-4-diamine-chemical-structure-title.png?v=1680597662&width=190)
![2,2-Bis[4-(4-aminophenoxy)phenyl]hexafluoropropane (4-BDAF)](http://www.ossila.com/cdn/shop/products/4-bdaf-chemical-structure-title.png?v=1681225583&width=190)
![1-[2-(Trifluoromethyl)phenyl]imidazole](http://www.ossila.com/cdn/shop/files/1-2-trifluoromethylphenylimidazole-chemical-structure-title.png?v=1682593257&width=190)