Vanadium Diselenide (VSe2) Powder and Crystal
CAS Number 12299-51-3
2D Materials, Low Dimensional Materials, Transition Metal Chalcogenides (TMCs), Transition Metal Dichalcogenides
Please choose the appropriate MSDS from the list below. Links will open in a new window. If your browser does not support PDFs, you will be prompted to download the file instead.
Low price, high purity 2D metal vanadium diselenide powder and crystals
For the development of next-generation electronics, optoelectronics, and nanotechnology
Vanadium diselenide (VSe2), CAS number 12299-51-3, is a TMDC material. With a sandwiched layer structure, its layers are bound by weak van de Waals forces which allow the isolation of each layer from its bulk crystals (or powder) to reduce the dimensionality - by either mechanical or chemical exfoliation.
In bulk form VSe2 is paramagnetic. It commonly condenses in the 1T structure, which is metallic with no optical bandgap. Monolayer VSe2 has magnetic characteristics and a lower work-function at its edge. However, strong room-temperature ferromagnetism has been observed in VSe2 monolayers grown on van der Waals substrates such as graphite.
High Purity
>99.999% Vanadium Diselenide Purity
Sheets
Used to produce single or few-layer sheets
Low Cost
Low Cost Vanadium Diselenide
Powder & Crystal
Different Forms of Vanadium Diselenide
We supply low price vanadium diselenide in several different forms for a range of applications.

Vanadium Diselenide Powder
Can be used for preparation of vanadium diselenide nanoplates and ultrathin films
Sold by weight
≥99.995% purity
From £350

Vanadium Diselenide Crystals by Size
Can be used to produce single or few-layer vanadium diselenide sheets via mechanical or liquid exfoliation
Small (≥10 mm2) or medium (≥25 mm2) crystals available*
≥99.999% purity
From £520
*Typical representative size, areas/dimensions may vary
Bulk single vanadium diselenide crystals are most commonly used as sources from which single or few-layer sheets can be obtained via either mechanical or liquid exfoliation. Single vanadium diselenide crystal or films produced from such crystals are suitable for study using atomic force microscopy or transmission electron microscopy
Platinum FET Test Chips for 2D Materials

- Affordable
- World-Wide Shipping
- Dual Channel Electrodes
Buy Online £200
Vanadium diselenide powder can also be used to prepare VSe2 nanosheets and nanoparticles by liquid-exfoliation (normally assisted by sonication), especially when it is the case of foreign elements such as lithium or sodium cations being inserted between layers by the process of intercalation. Liquid exfoliation can provide mass production of such products.
Key Product Data
- High purity, low price vanadium diselenide
- Available as a powder or as individual crystals
- Can be used to produce single or few-layer sheets
- Free worldwide shipping on qualifying orders
Structure of Vanadium Diselenide
In nature, vanadium diselenide (VSe2) exists only in 1T as its thermodynamically-stable phase (space group: P63mc). Single-layer VSe2 film consists of stacks of one layer of hexagonally close-packed transition metal vanadium atoms sandwiched between two layers of chalcogen selenium atoms in the sequence of Se–V–Se. The metal atoms are octahedrally coordinated and covalently bonded to the chalcogen atoms. The layers are stacked together by van der Waals (vdW) forces. Like black phosphorus and MoSe2, these can be exfoliated into 2D thin layers.
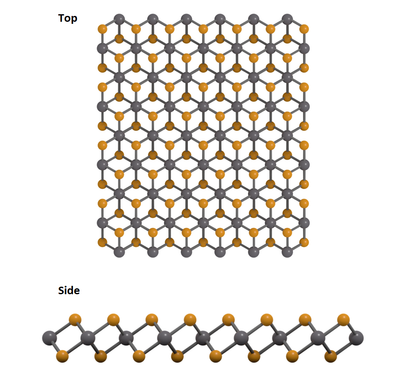
Properties of 2D Vanadium Diselenide
After exfoliation of crystals or powder, vanadium diselenide typically has the following properties:
- Octahedral (1T) structure (space group: P63mc)
- Bulk 1T-VSe2 is a metal and has no bandgap
- VSe2 shows unique three-dimensional (3D) CDW properties below 110K and thickness dependence of the CDW transition temperature (Tp)
- Monolayer VSe2 has magnetic characteristics and a lower work-function at its edge
Applications of Vanadium Diselenide
Vanadium diselenide single crystals can be used to prepare monolayer and few-layer VSe2 by mechanical or liquid exfoliation.
Vanadium diselenide powder is suitable for liquid chemical exfoliation to prepare VSe2 nanosheets and nanoparticles down to few-layer films. 2D VSe2 nanosheets (NSs) can be successfully produced via an ultrasonication-assisted lithium intercalation process using lithium carbonate in a colloidal solution of NPs. VSe2 powder is also used for the preparation of mono-layer and few-layer films via chemical vapour deposition (CVD).
Vanadium diselenide powder is also used to study its electronic and heat transport, electronic band structure and charge-density wave (CDW) properties with alkali-metal-intercalated 2D VSe2 heterostructures.
The metallic properties of vanadium diselenide contribute to its potential for applications in supercapacitors and lithium-ion and sodium-ion batteries such as electrodes, moisture-detection sensors, and electrocatalysts for hydrogen revolution reactions (HER).
Literature and Reviews
- Field-Effect Tuned Adsorption Dynamics of VSe2 Nanosheets for Enhanced Hydrogen Evolution Reaction, M. Yan et al., Nano Lett. 2017, 17, 4109−4115 (2017); DOI: 10.1021/acs.nanolett.7b00855.
- Electronic Structure and Enhanced Charge-Density Wave Order of Monolayer VSe2, J. Feng et al., Nano Lett., 18, 4493−4499 (2018); DOI: 10.1021/acs.nanolett.8b01649.
- Emergence of a Metal−Insulator Transition and High-Temperature Charge-Density Waves in VSe2 at the Monolayer Limit, G, Duvjir et al., Nano Lett. 2018, 18, 5432−5438 (2018); DOI: 10.1021/acs.nanolett.8b01764.
- Chemical Vapor Deposition of 2D Vanadium Disulfide and Diselenide and Raman Characterization of the Phase Transitions, M. Hossain et al, Adv. Mater. Interfaces, 5, 1800528 (2018); DOI: 10.1002/admi.201800528.
- Versatile Electronic Properties of VSe2 Bulk, Few-Layers, Monolayer, Nanoribbons, and Nanotubes: A Computational Exploration, F. Li et al., J. Phys. Chem. C, 118, 21264−21274 (2014); doi: 10.1021/jp507093t.
- Strong room-temperature ferromagnetism in VSe2 monolayers on van der Waals substrates, M. Bonilla et al., Nat.Nanotech., 13, 289–293 (2018); DIO: 10.1038/s41565-018-0063-9.
- Thickness dependence of the chargedensity-wave transition temperature in VSe2, J. Yang et al., Appl. Phys. Lett. 105, 063109 (2014); DIO: 10.1063/1.4893027.
- Strain and interlayer coupling tailored magnetic properties and valley splitting in layered ferrovalley 2H-VSe2, S. Feng et al., Appl. Surf. Sci., 458, 191–197 (2018); DIO: 10.1016/j.apsusc.2018.07.070.
- Epitaxially grown monolayer VSe2: an air-stable magnetic two-dimensional material with low work function at edges, Z. Liu et al., Sci. Bull., 63, 419–425 (2018); DIO: 10.1016/j.scib.2018.03.008.
- Intrinsic valley polarization of magnetic VSe2 monolayers, J. Liu et al., J. Phys.: Condens. Matter, 29, 255501 (2017); DIO: 10.1088/1361-648X/aa6e6e.
- Dimensional crossover of the charge density wave transition in thin exfoliated VSe2, A. Pásztor et al., 2D Mater., 4, 041005 (2017); DIO: 10.1088/2053-1583/aa86de.
- Metallic Graphene-Like VSe2 Ultrathin Nanosheets: Superior Potassium-Ion Storage and Their Working Mechanism, C. Yang et al., Adv. Mater., 30, 1800036 (2018); DOI: 10.1002/adma.201800036.
- Catalytic Properties of Vanadium Diselenide: A Comprehensive Study on Its Electrocatalytic Performance in Alkaline, Neutral, and Acidic Media, T. Ghobadi et al., ACS Omega, 2, 8319−8329 (2017); DOI: 10.1021/acsomega.7b01226.
Technical Data
| CAS Number | 12299-51-3 |
| Chemical Formula | VSe2 |
| Molecular Weight | 208.86 g/mol |
| Bandgap | ~1.4 eV (indirect) |
| Preparation | Synthetic - Chemical Vapour Transport (CVT) |
| Structure | Octahedral (1T) |
| Electronic Properties | Metal |
| Melting Point | N/A |
| Colour | Dark brown |
| Synonyms | Vanadium selenide, Bis(selanylidene)vanadium |
| Classification / Family | Transition Metal Dichalcogenides (TMDCs), 2D Semiconductor materials, Charge Density Wave (CDW), Hydrogen Revolution Reactions (HER), Nano-electronics, Nano-photonics, Photovoltaic, Materials science |
Product Details
| Form | Purity |
| Powder | ≥99.995% |
| Crystal | ≥99.999% |
MSDS Documents
Pricing Table
| Product Code | Form | Size/Weight* | Price |
| M2154C1 | Powder | 1 g | £350 |
| M2154A10 | Crystal | Small (≥10 mm2) | £520 ea. |
| M2154A25 | Crystal | Medium (≥25 mm2) | £850 ea. |
*typical representative size, areas/dimensions may vary

 Vanadium diselenide powder
Vanadium diselenide powder

















































































