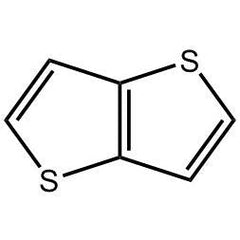
Thienothiophene, Thieno[3,2-b]thiophene
CAS Number 251-41-2
Chemistry Building Blocks, Heterocyclic Building Blocks, Monomers
Thienothiophene, for the synthesis of small molecules and polymers
Available online for fast, secure dispatch
Thieno[3,2-b]thiophene (CAS number 251-41-2), also known as thienothiophene (TT), is a heterocyclic compound. It belongs to the family of fused thiophenes and is widely used as an intermediate for the synthesis of small molecules and polymers in the application of organic field-effect transistors (OFETs) and organic photovoltaic devices (OPV).
Fused thiophenes are more electron-rich and more structurally rigid, with extended π-conjugation so they are good candidates for adjusting the band gap of the organic polymer semiconducting materials, i.e. Poly[2,5-bis(3-hexadecylthiophen-2-yl)thieno[3,2-b]thiophene] (also known as PBTTT).
![pbttt synthesis with 2,5-bis(trimethylstannyl)thieno[3,2-b]thiophene and 5,5'-dibromo-4,4'-didodecyl-2,2'-bithiophene or 5,5'-dibromo-4,4'-dihexadecyl-2,2'-bithiophene or 5,5'-dibromo-4,4'-ditetradecyl-2,2'-bithiophene](http://cdn.shopify.com/s/files/1/0823/0287/files/pbttt-synthesis_636x148.jpg?6038470244592440286)
General Information
| CAS Number | 251-41-2 |
| Chemical Formula | C6H4S2 |
| Molecular Weight | 140.23 g/mol |
| Synonyms |
1,4-Dithiapentalene Thienothiophene |
| Classification / Family | Thiophene, Fused thiophene, Dithiapentalene, Heterocylic aromatics, Five-membered ring, Semiconductor synthesis intermediates, Low band gap polymers OFETs, Organic Photovoltaics, Polymer solar cells |
Chemical Structure
Product Details
| Purity | >95% |
| Melting Point | 56 °C - 58 °C |
| Appearance | White crystals |
MSDS Documentation
![Thieno[3,2-b]thiophene MSDS](https://cdn.shopify.com/s/files/1/0823/0287/files/msds-sheets_60x60.jpg) Thieno[3,2-b]thiophene MSDS sheet
Thieno[3,2-b]thiophene MSDS sheet
Literature and Reviews
- Thienothiophenes, Dithienothiophenes, and Thienoacenes: Syntheses, Oligomers, Polymers, and Properties, M. E. Cinar et al., Chem. Rev., (2015), DOI: 10.1021/cr500271a
- A stable solution-processed polymer semiconductor with record high-mobility for printed transistors, Li et al., Sci. Reports, 2, 754, (2012) DOI: 10.1038/srep00754.
- Liquid-crystalline semiconducting polymers with high charge-carrier mobility, I. McCulloch et al., Nat. Mater., 5, 328 (2006)
Related Products
We stock a wide range of monomers available to purchase online. Please contact us if you cannot find what you are looking for.
![Thienothiophene, Thieno[3,2-b]thiophene CAS 251-41-2](http://www.ossila.com/cdn/shop/products/thienothiophene.jpg?v=1431611114&width=240)
![2-Ethylhexyl 4,6-dibromo-3-fluorothieno[3,4-b]thiophene-2-carboxylate](http://www.ossila.com/cdn/shop/products/ptb7-monomer-b361-ossila-chemical-structure.png?v=1648818400&width=190)
![2,5-bis(trimethylstannyl)-thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/2_5-bis-trimethylstannyl-thieno-3_2-b-thiophene_structure.jpg?v=1504193831&width=190)
![2,6-dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/4Hcyclopentadithiophene.jpg?v=1431610575&width=190)
![2,7-Dibromo-9,9-bis[3,3'-(N,N-dimethylamino)-propyl]fluorene](http://www.ossila.com/cdn/shop/products/dibromo-fluorene-diyl-bisdimethylpropan-amine.jpg?v=1431610994&width=190)
![3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione](http://www.ossila.com/cdn/shop/products/bisbromothiophenyl-bisoctyldodecylpyrrolo-dione.jpg?v=1431611190&width=190)
![4H-Cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/cyclopentadithiophene_12a14774-f96a-4ebc-a0d0-2c5483da9180.jpg?v=1445441165&width=190)
![Benzo[1,2-b:4,5-b']dithiophene-4,8-dione](http://www.ossila.com/cdn/shop/products/benzo-dithiophene-dione.jpg?v=1437904702&width=190)
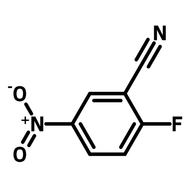
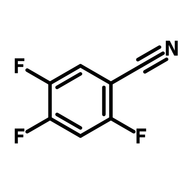
![Thieno[3,2-b]thiophene-2-carbonitrile](http://www.ossila.com/cdn/shop/products/thienothiophene-2-carbonitrile.jpg?v=1439548051&width=190)
![DTT, Dithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/dtt-chemical-structure.png?v=1653477307&width=190)
![3,6-Dibromothieno[3,2-b]thiophene (TT36)](http://www.ossila.com/cdn/shop/products/3-6-dibromothienothiophene-chemical-structure.png?v=1653663075&width=190)
![2,3,5,6-Tetrabromothieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/Tetrabromo-thienothiophene-chemical-structure.png?v=1665673773&width=190)
![2,6-Dibromodithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/Dibromodithienothiophene-chemical-structure.png?v=1666702461&width=190)
![2,5-Dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c]pyrrole-1,4-dione](http://www.ossila.com/cdn/shop/products/2_5-Dihydro-3_6-di-2-thienyl-pyrrolo_3_4-c_pyrrole-1_4-dione-chemical-structure-dpp.png?v=1667321819&width=190)
![6,9-bis(5-bromo-4-(2-butyloctyl)thiophen-2-yl)dithieno[3,2-f:2',3'-h]quinoxaline](http://www.ossila.com/cdn/shop/products/bisbromo-butyloctylthiophenyl-dithienoquinoxaline-chemical-structure.png?v=1669202898&width=190)
![Indolo[3,2-b]carbazole](http://www.ossila.com/cdn/shop/products/Indolocarbazole-chemical-structure.png?v=1670495077&width=190)
![10,15-Dihydro-5H-diindolo[3,2-a:3',2'-c]carbazole](http://www.ossila.com/cdn/shop/products/Dihydro-diindolocarbazole-chemical-structure.png?v=1670502109&width=190)
![Indolo[2,3-a]carbazole](http://www.ossila.com/cdn/shop/products/indolo-2-3-a-carbazole-chemical-structure-title.png?v=1678288567&width=190)
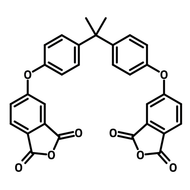
![2,2-Bis[4-(4-aminophenoxy)phenyl]propane (BAPP)](http://www.ossila.com/cdn/shop/products/bapp-chemical-structure-title.png?v=1679403349&width=190)
![2,2'-Dimethyl[1,1'-biphenyl]-4,4'-diamine](http://www.ossila.com/cdn/shop/products/2-2-dimethyl1-1-biphenyl-4-4-diamine-chemical-structure-title.png?v=1680597662&width=190)
![2,2-Bis[4-(4-aminophenoxy)phenyl]hexafluoropropane (4-BDAF)](http://www.ossila.com/cdn/shop/products/4-bdaf-chemical-structure-title.png?v=1681225583&width=190)
![1-[2-(Trifluoromethyl)phenyl]imidazole](http://www.ossila.com/cdn/shop/files/1-2-trifluoromethylphenylimidazole-chemical-structure-title.png?v=1682593257&width=190)