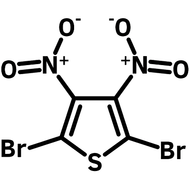
2,3,5,6-Tetrabromothieno[3,2-b]thiophene
CAS Number 124638-53-5
Chemistry Building Blocks, Heterocyclic Building Blocks, Monomers
2,3,5,6-Tetrabromothieno[3,2-b]thiophene
With functionalities spreading over the fused thienothiophene ring, this intermediate is widely used for the synthesis of supramolecules, semiconducting molecules, oligomers and conjugated polymers.
2,3,5,6-Tetrabromothieno[3,2-b]thiophene, CAS number 124638-53-5, is an all brominated thieno[3,2-b]thiophene derivative. 2,3,5,6-tetrabromothieno[3,2-b]thiophene is an intermediate with full functionality around the fused rings to extend its conjugation to more complex structures such as conjugated polymers, supramolecules, ladder type polythienothiophenes for OFETs and OPV applications. 2,3,5,6-Tetrabromothieno[3,2-b]thiophene can be synthesised by full bromination of thienothiophene by using bromine as the oxidising agent in acetic acid and chloroform. Large scale synthesis can be achieved by bromination of thieno[3,2-b]thiophene-2-carboxylic acid with bromine in water and acetic acid.
![The synthesis of 2,3,5,6-Tetrabromothieno[3,2-b]thiophene](https://cdn.shopify.com/s/files/1/0823/0287/files/tetrabromothioenothiophene-synthesis_560x135.png?v=1665744878)
2,3,5,6-Tetrabromothieno[3,2-b]thiophene can be selectively reduced to 3,6-dibromothieno[3,2-b]thiophene by using zinc in acetic acid. Further function at 3.6-positions can give ladder type poly(thienothiophenes) to extend conjugations to the fused thiophene rings. Electropolymerization of 3,6-dimethoxy-thieno[3,2-b]thiophen leads to a conjugated polymer with similar electronic and physical properties, i.e., redox potential, bandgap, optical transparency and stability to PEDOT.
General Information
| CAS Number | 124638-53-5 |
| Chemical Formula | C6Br4S2 |
| Full Name | 2,3,5,6-Tetrabromothieno[3,2-b]thiophene |
| Molecular Weight | 455.80 g/mol |
| Synonyms | Perbromothieno[3,2-b]thiophene, tetrabromo-thieno[3,2-b]thiophene |
| Classification / Family | Thienothiophene, semiconductor synthesis intermediates, low band gap polymers, OLED, OFETs, organic photovoltaics |
Chemical Structure
![3,6-Dibromothieno[3,2-b]thiophene (TT36) chemical structure, CAS 124638-53-5](https://cdn.shopify.com/s/files/1/0823/0287/files/Tetrabromo-thienothiophene-chemical-structure-body_240x180.png?v=1665674568)
Product Details
| Purity | >98% (HPLC) |
| Melting Point | 234.0 °C |
| Appearance | White powder/crystals |
MSDS Documentation
![2,3,5,6-tetrabromothieno[3,2-b]thiophene MSDS](https://cdn.shopify.com/s/files/1/0823/0287/files/msds-sheets_60x60.jpg) 2,3,5,6-Tetrabromothieno[3,2-b]thiophene MSDS Sheet
2,3,5,6-Tetrabromothieno[3,2-b]thiophene MSDS Sheet
Literature and Reviews
- Thienothiophenes. Part 2.1 Synthesis, metallation and bromine→lithium exchange reactions of thieno[3,2-b]thiophene and its polybromo derivatives, L. Fuller et al., J. Chem. Soc., Perkin Trans. 1, 3465-3470 (1997); DOI: 10.1039/A701877K.
- Poly(3,6-dimethoxy-thieno[3,2-b]thiophene): a possible alternative to poly(3,4-ethylenedioxythiophene) (PEDOT), M. Turbiez et al., Chem. Commun., 1161-1163 (2005); DOI: 10.1039/B414822C.
- High-performance organic field-effect transistors based on single-crystalline microribbons of a two-dimensional fused heteroarene semiconductor, Y. Wang et al., Chem. Commun., 51, 11961-11963 (2015); 10.1039/C5CC03305E.
- Ullmann-Type Intramolecular C−O Reaction Toward Thieno[3,2‑b]furan Derivatives with up to Six Fused Rings, D. Chen et al., J. Org. Chem., 82, 20, 10920–10927 (2017); DOI: 10.1021/acs.joc.7b01745.
- Versatile α, ω -Disubstituted Tetrathienoacene Semiconductors for High Performance Organic Thin-Film Transistors, J. Youn et al., Adv. Funct. Mater., 22, 48-60 (2011); DOI: 10.1002/adfm. 201101053.
- High-Performance n-Channel Field Effect Transistors Based on Solution-Processed Dicyanomethylene-Substituted Tetrathienoquinoid, Q. Wu et al., RSC Adv., 4, 16939-16943 (2014); 10.1039/C3RA47095D.
- Antiaromatic Bisindeno-[n]thienoacenes With Small Singlet Biradical Characters: Syntheses, Structures and Chain Length Dependent Physical Properties, X. Shi et al., Chem. Sci., 5, 4490-4503 (2014); DOI: 10.1039/C4SC01769B.
- General Synthesis of Extended Fused Oligothiophenes Consisting of Even Number of Thiophene Rings, T. Okamoto et al., Chem. Eur. J, 13(2), 548-56 (2006); DOI: 10.1002/chem.200601064.
Related Products
We stock a wide range of monomers available to purchase online. Please contact us if you cannot find what you are looking for.
![2,3,5,6-Tetrabromothieno[3,2-b]thiophene CAS 124638-53-5](http://www.ossila.com/cdn/shop/products/Tetrabromo-thienothiophene-chemical-structure.png?v=1665673773&width=240)
![2-Ethylhexyl 4,6-dibromo-3-fluorothieno[3,4-b]thiophene-2-carboxylate](http://www.ossila.com/cdn/shop/products/ptb7-monomer-b361-ossila-chemical-structure.png?v=1648818400&width=190)
![2,5-bis(trimethylstannyl)-thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/2_5-bis-trimethylstannyl-thieno-3_2-b-thiophene_structure.jpg?v=1504193831&width=190)
![2,6-dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/4Hcyclopentadithiophene.jpg?v=1431610575&width=190)
![2,7-Dibromo-9,9-bis[3,3'-(N,N-dimethylamino)-propyl]fluorene](http://www.ossila.com/cdn/shop/products/dibromo-fluorene-diyl-bisdimethylpropan-amine.jpg?v=1431610994&width=190)
![3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione](http://www.ossila.com/cdn/shop/products/bisbromothiophenyl-bisoctyldodecylpyrrolo-dione.jpg?v=1431611190&width=190)
![4H-Cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/cyclopentadithiophene_12a14774-f96a-4ebc-a0d0-2c5483da9180.jpg?v=1445441165&width=190)
![Benzo[1,2-b:4,5-b']dithiophene-4,8-dione](http://www.ossila.com/cdn/shop/products/benzo-dithiophene-dione.jpg?v=1437904702&width=190)
![Thienothiophene, Thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/thienothiophene.jpg?v=1431611114&width=190)
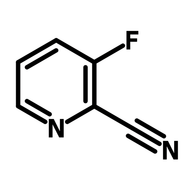
![Thieno[3,2-b]thiophene-2-carbonitrile](http://www.ossila.com/cdn/shop/products/thienothiophene-2-carbonitrile.jpg?v=1439548051&width=190)
![DTT, Dithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/dtt-chemical-structure.png?v=1653477307&width=190)
![3,6-Dibromothieno[3,2-b]thiophene (TT36)](http://www.ossila.com/cdn/shop/products/3-6-dibromothienothiophene-chemical-structure.png?v=1653663075&width=190)
![2,6-Dibromodithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/Dibromodithienothiophene-chemical-structure.png?v=1666702461&width=190)
![2,5-Dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c]pyrrole-1,4-dione](http://www.ossila.com/cdn/shop/products/2_5-Dihydro-3_6-di-2-thienyl-pyrrolo_3_4-c_pyrrole-1_4-dione-chemical-structure-dpp.png?v=1667321819&width=190)
![6,9-bis(5-bromo-4-(2-butyloctyl)thiophen-2-yl)dithieno[3,2-f:2',3'-h]quinoxaline](http://www.ossila.com/cdn/shop/products/bisbromo-butyloctylthiophenyl-dithienoquinoxaline-chemical-structure.png?v=1669202898&width=190)
![Indolo[3,2-b]carbazole](http://www.ossila.com/cdn/shop/products/Indolocarbazole-chemical-structure.png?v=1670495077&width=190)
![10,15-Dihydro-5H-diindolo[3,2-a:3',2'-c]carbazole](http://www.ossila.com/cdn/shop/products/Dihydro-diindolocarbazole-chemical-structure.png?v=1670502109&width=190)
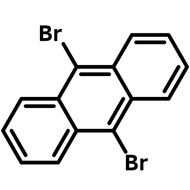
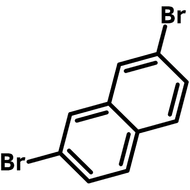
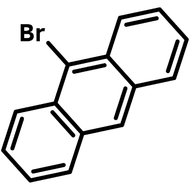
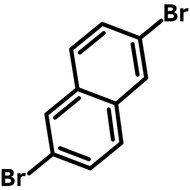
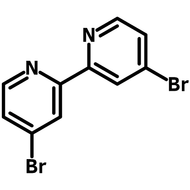
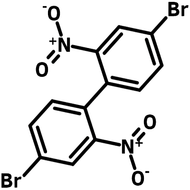
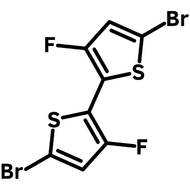
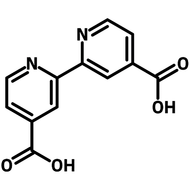
![Indolo[2,3-a]carbazole](http://www.ossila.com/cdn/shop/products/indolo-2-3-a-carbazole-chemical-structure-title.png?v=1678288567&width=190)
![2,2-Bis[4-(4-aminophenoxy)phenyl]propane (BAPP)](http://www.ossila.com/cdn/shop/products/bapp-chemical-structure-title.png?v=1679403349&width=190)
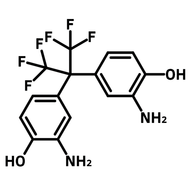
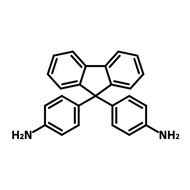
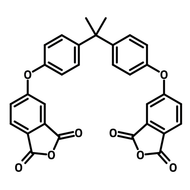
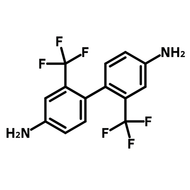
![2,2'-Dimethyl[1,1'-biphenyl]-4,4'-diamine](http://www.ossila.com/cdn/shop/products/2-2-dimethyl1-1-biphenyl-4-4-diamine-chemical-structure-title.png?v=1680597662&width=190)
![2,2-Bis[4-(4-aminophenoxy)phenyl]hexafluoropropane (4-BDAF)](http://www.ossila.com/cdn/shop/products/4-bdaf-chemical-structure-title.png?v=1681225583&width=190)
![1-[2-(Trifluoromethyl)phenyl]imidazole](http://www.ossila.com/cdn/shop/files/1-2-trifluoromethylphenylimidazole-chemical-structure-title.png?v=1682593257&width=190)