PSBTBT
CAS Number 1089687-02-4
OFET & OLED Polymer Materials, OPV Polymers, Perovskite Interface Materials, Perovskite Materials, Semiconducting Polymers
PSBTBT (CAS number 1089687-02-4), also known as Si-PCPDTBT, is a low band-gap polymer semiconductor that has a large absorption band in the visible light spectrum. It also extends its absorption to near-IR region.
PSBTBT is semi-crystalline with a shorter π-π stacking distance due to its significantly longer C-Si bond. This reduces the steric hindrance between the bulky side chains and adjacent thiophene rings.
General Information
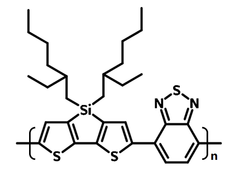
| Full name | Poly[(4,4‐bis(2‐ethylhexyl)‐dithieno[3,2‐b:2′,3′‐d]silole)‐2,6‐diyl‐alt‐(2,1,3‐benzothiadiazole)‐4,7‐diyl] |
| Synonyms | Si-PCPDTBT |
| CAS number | 1089687-02-4 |
| Chemical formula | (C30H38N2S3Si)n |
| Molecular weight | See Batch Details table above |
| HOMO / LUMO | HOMO = - 5.0 eV, LUMO = - 3.5 eV [1] |
| Solubility | Chloroform, chlorobenzene, dichlorobenzene |
| Classification / Family | Dithienosilole, Organic semiconducting materials, Low band gap polymers, Organic photovoltaics, All-polymer solar cells, OFETs, Photodetectors |
Chemical Structure
MSDS Documentation
Pricing
| Batch | Quantity | Price |
| M2095A2/A3 | 100 mg | £380 |
| M2095A2/A3 | 250 mg | £800 |
| M2095A2/A3 | 500 mg | £1500 |
| M2095A2/A3 | 1 g | £2600 |
Batch Details
| Batch | Mw | Mn | PDI | Stock Info |
| M2095A1 | 95 kDa | 50,000 | 1.9 | Discontinued |
| M2095A2 | 95 kDa | 45,238 | 2.1 | In stock |
| M2095A3 | 25,001 | 12,933 | 1.93 | In Stock |
Literature and Reviews
- 8.91% Power Conversion Efficiency for Polymer Tandem Solar Cells, Abd. Rashid bin Mohd Yusoff et al., Adv. Funct. Mater., 24, 2240–2247 (2014); DOI: 10.1002/adfm.201303471.
- Efficient Exciton Harvesting through Long-Range Energy Transfer, Y. Wang et al., ChemPhysChem, 16, 1263–1267 (2015); DOI: 10.1002/cphc.201402740.
- Sub-ns triplet state formation by non-geminate recombination in PSBTBT:PC70BM and PCPDTBT:PC60BM organic solar cells, F. Etzold et al., Energy Environ. Sci., 2015, 8, 1511-1522 (2015); DOI: 10.1039/C4EE03630A (Paper)
- High-Performance Flexible Tandem Polymer Solar Cell Employing a Novel Cross-Linked Conductive Fullerene as an Electron Transport Layer, C-Y. Chang et al., Chem. Mater., 2015, 27 (5), pp 1869–1875; DOI: 10.1021/acs.chemmater.5b00161.
- What To Expect from Conducting Polymers on the Playground of Thermoelectricity: Lessons Learned from Four High-Mobility Polymeric Semiconductors. Q. Zhang et al., Macromolecules, 47 (2), 609–615 (2014); DOI: 10.1021/ma4020406.
 PSBTBT MSDS sheet
PSBTBT MSDS sheet