PNDI(2OD)2T
CAS Number 1100243-40-0
Luminosyn™ Polymers, OFET & OLED Polymer Materials, OPV Polymers, Semiconducting Polymers
P(NDI2OD-T2), electron acceptor in polymer solar cells
High mobility n-type polymer semiconductor available online for priority dispatch
PNDI(2OD)2T (CAS number 1100243-40-0), a copolymer of naphthalene diimide (NDI) and bithiophene unit, has been intensively studied for use as an electron acceptor in polymer solar cells. This is due to its high electron mobility, high electron affinity, and broad light absorption. All polymer solar cells with PNDI(2OD)2T as an acceptor and J51 as a donor (fullerene-free) have demonstrated a power conversion efficiency over 8% [1].
PNDI(2OD)2T is also known as a high-mobility n-type polymer semiconductor. PNDI(2OD)2T-based OFET devices have electron mobilities up to 0.45–0.85 cm2V-1 s-1[2].
PNDI(2OD)2T from Ossila was used in the high-impact paper (IF 18.81), Tuning Contact Resistance in Top-Contact p-Type and n-Type Organic Field Effect Transistors by Self-Generated Interlayers, T. Sarka et al., Adv. Funct. Mater., 1805617 (2020); DOI: 10.1002/adfm.201805617.
The synthesis of the polymer P(NDI2OD-T2) is described here and we also have PNDI(2HD)T, PNDI(2HD)T2 and PNDI(2OD)2T-2F available.
Luminosyn™ PNDI(2OD)2T
Luminosyn™ PNDI(2OD)2T is now available.
High molecular weight and high purity
PNDI(2OD)2T is purified by Soxhlet extraction with methanol, hexane and chlorobenzene under an argon atmosphere
Batch-specific GPC data
Have confidence in what you are ordering; batch-specific GPC data for your thesis or publications
Large quantity orders
Plan your experiments with confidence with polymers from the same batch
General Information
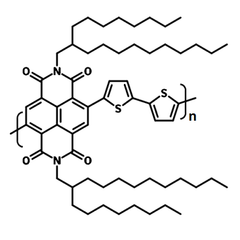
| Full name | Poly{[N,N'-bis(2-octyldodecyl)naphthalene-1,4,5,8-bis(dicarboximide)-2,6-diyl]-alt-5,5'-(2,2'-bithiophene)} |
| Synonyms |
|
| Chemical formula | (C62H88N2O4S2)n |
| CAS number | 1100243-40-0 |
| HOMO / LUMO | HOMO = -5.77 eV, LUMO = -3.84 eV [1] |
| Classification / Family | PNDI polymers, Organic n-type semiconducting materials, Organic photovoltaics, Polymer solar cells, Electron-acceptor polymers, OFETs, Perovskite solar cells. |
| Solubility | Soluble in chloroform, chlorobenzene, dichlorobenzene |
Chemical Structure

MSDS Documentation
Pricing
| Batch | Quantity | Price |
| M1201A | 100 mg | £400 |
| M1201A | 250 mg | £800 |
| M1201A | 500 mg | £1450 |
| M1201A | 1 g | £2500 |
Batch details
| Batch | Mw | Mn | PDI | Stock Info |
| M1201 | ≥30,000 | ≤3.0 | Discontinued | |
| M1202 | 168,138 | 92,338 | 1.82 | Discontinued |
| M1203 | 289,730 | 150,497 | 1.93 | Discontinued |
| M1204 | 123,320 | 67,167 | 1.84 | Discontinued |
| M1201A1 | 132,276 | 31,079 | 4.26 | Discontinued |
| M1201A2 | 125,059 | 54,373 | 2.3 | Discontinued |
| M1201A3 | 202,261 | 90,982 | 2.22 | Discontinued |
| M1201A4 | 186,027 | 90,949 | 2.05 | In Stock |
Literature and Reviews
- All-Polymer Solar Cells Based on Absorption-Complementary Polymer Donor and Acceptor with High Power Conversion Efficiency of 8.27%, L. Gao et al., Adv. Mater., 28, 1884–1890 (2016); DOI: 10.1002/adma.201504629.
- A high-mobility electron-transporting polymer for printed transistors, H. Yan et al., Nature, 457 (2009); doi:10.1038/nature07727.
- Highly efficient charge-carrier generation and collection in polymer/polymer blend solar cells with a power conversion efficiency of 5.7%, D. Mori et al., Energy Environ. Sci., 7, 2939-2943 (2014); DOI: 10.1039/C4EE01326C.
- Bulk Electron Transport and Charge Injection in a High Mobility n-Type Semiconducting Polymer, R. Steyrleuthner et al., Adv. Mater., 22, 2799–2803 (2010); DOI: 10.1002/adma.201000232.n2200.
- High-performance ternary blend all-polymer solar cells with complementary absorption bands from visible to near-infrared wavelengths, H. Benten et al., Energy Environ. Sci., 9, 135-140 (2016); DOI: 10.1039/C5EE03460D.
- High efficiency all-polymer tandem solar cells, J. Yuan et al., Sci. Reports 6, 26459 (2016); doi:10.1038/srep26459.
- Regioregular narrow-bandgap-conjugated polymers for plastic electronics, L. Ying et al., Nature Commun., 8:14047 (2017); DOI: 10.1038/ncomms14047.
- Naphthalene Diimide-Based Polymer Semiconductors: Synthesis, Structure−Property Correlations, and n-Channel and Ambipolar Field-Effect Transistors, X. Guo et al., Chem. Mater., 24, 1434−1442 (2012); DOI: 10.1021/cm2034273.
 PNDI(2OD)2T MSDS sheet
PNDI(2OD)2T MSDS sheet