L8-BO, L8-BO-2F
CAS Number 2668341-40-8
Green Energy Materials, Non-Fullerene Acceptors, Organic Conductors
L8-BO, non-fullerene acceptor for polymer solar cells
For high carrier generation and balanced charge transport
L8-BO (CAS number 2668341-40-8) has a similar structure to the highly efficient Y6 non-fullerene acceptor with a fused thienothienopyrrolo-thienothienoindole (TTP-TTI) core. The only difference between L8-BO and Y6 is that L8-BO has two butyloctyl side chains attached to the core while Y6 has two undecyl side chains. The branched 2-butyloctyl side chains show great improvement in film while comparing with Y6 with better structural order to achieve an optimized, multi-length-scale film morphology. High carrier generation, low charge recombination rates and balanced charge transport can be expected.
High efficient non-flullerene acceptor
With highly conjugated core
Improved film quanlity
Owing to the branched butyloctyl side chain
Worldwide shipping
Quick and reliable shipping
High purity
>99% pure
It has been reported that single-junction non-fullerene polymer solar cells with L8-BO as the acceptor and PBDB-T-2F (PM6) as the polymer has achieved photo conversion efficiency (PCE) over 18% with the following device structure.
Device structure: ITO/PEDOT:PSS/PM6:L8-BO (1:1.2)/PNDIT-F3N/Ag
| Thickness (nm) | VOC (V) | JSC (mA cm-2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| 100 | 0.874 | 25.72 | 81.5 | 18.32 |
General Information
| CAS Number | 2668341-40-8 |
|---|---|
| Chemical Formula | C84H90F4N8O2S5 |
| Purity | >99% (1H NMR) |
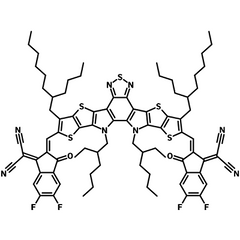
| Full Name | 2,2'-((2Z,2'Z)-((12,13-bis(2-ethylhexyl)-3,9-(2-butyloctyl)-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2",3’':4’,5']thieno[2',3':4,5]pyrrolo[3,2-g]thieno[2',3':4,5]thieno[3,2-b]indole-2,10-diyl)bis(methanylylidene))bis(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile |
| Molecular Weight | 1479.98 g/mol |
| HOMO / LUMO | HOMO = -5.68 eV, LUMO = -3.90 eV [1] |
| Solubility | Chloroform, chlorobenzene and dichlorobenzene |
| Form | Dark blue powder/crystal |
| Synonyms | L8BO, L8-BO-2F |
| Classification / Family | BTP series NFAs, n-type non-fullerene electron acceptors, Organic semiconducting materials, Low band-gap small molecule, Small molecular acceptor, Organic photovoltaics, Polymer solar cells, NF-PSCs. |
Chemical Structure

MSDS Documentation
Pricing
| Batch | Quantity | Price |
|---|---|---|
| M2304A1 | 50 mg | £260 |
| M2304A1 | 100 mg | £400 |
| M2304A1 | 250 mg | £800 |
| M2304A1 | 500 mg | £1450 |
| M2304A1 | 1 g | £2500 |
| M2304A1 | 5 g / 10 g* | Please contact us for details |
*for 5 – 10 grams order quantity, the lead time is 4 – 6 weeks
Literature and Reviews
- Non-fullerene acceptors with branched side chains and improved molecular packing to exceed 18% efficiency in organic solar cells, C. Li et al., Nat. Energy, 6, 605–613 (2021); DOI: 10.1038/s41560-021-00820-x.
- High-efficiency organic solar cells with low voltage loss induced by solvent additive strategy, J. Song et al., Matter, 4 (7), 2542-2552 (2021); DOI: 10.1016/j.matt.2021.06.010.
- 18.77 % Efficiency Organic Solar Cells Promoted by Aqueous Solution Processed Cobalt(II) Acetate Hole Transporting Layer, H. Meng et al., Angew. Chem, 133 (41); 22728-22735 (2021); DOI: 10.1002/ange.202110550.
 L8-BO MSDS sheet
L8-BO MSDS sheet