CBP, 4,4′-Bis(N-carbazolyl)-1,1′-biphenyl
CAS Number 58328-31-7
High Purity Sublimed Materials, Host Materials, Perovskite Interface Materials, Perovskite Materials, Semiconducting Molecules, TADF Materials
CBP, host material for efficient OLEDs
Low price and available for priority dispatch
4,4′-Bis(N-carbazolyl)-1,1′-biphenyl (CBP), is one of the most widely-used host materials for efficient fluorescent and phosphorescent organic light-emitting diodes with high hole mobility. This is due to its electron-rich property from two carbazolyl units.
CBP, 4,4′-Bis(N-carbazolyl)-1,1′-biphenyl from Ossila was used in the high-impact paper (IF 14.92), Complete polarization of electronic spins in OLEDs, T. Scharff et al., Nat. Commun., 12, 2071 (2021); DOI: 10.1038/s41467-021-22191-3.
It has been demonstrated that CBP can efficiently host green, yellow and red phosphorescent emitters with triplet energies smaller than that of CBP (ET = 2.6 eV) [1].
General Information
| CAS number | 58328-31-7 |
|---|---|
| Chemical formula | C36H24N2 |
| Molecular weight | 484.59 g/mol |
| HOMO/LUMO | HOMO 6.0 eV, LUMO 2.9 eV |
| Synonyms | CBP, 4,4′-Bis(9-carbazolyl)-1,1′-biphenyl, 4,4-N,N′-Dicarbazole-1,1′-biphenyl,DCBP, BSBCz, BSB-Cz |
| Classification / Family |
Carbazole derivatives, Hole-injection layer materials, Hole transport layer materials, Hole blocking layer materials, Phosphorescent host materials, Light-emitting fiodes, Organic electronics, Sublimed materials |
Product Details
| Purity |
> 99.5% (sublimed) > 98.0% (unsublimed) |
|---|---|
| Melting point | 281-285 (lit.) °C |
| Appearance | White powder |
*Sublimation is a technique used to obtain ultra pure-grade chemicals. For more details about sublimation, please refer to the Sublimed Materials page.
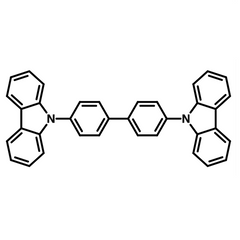
Chemical Structure

Device Structure(s)
| Device structure | ITO/MoO3 (3 nm)/CBP: 20 wt% Ir(ppy)3: 4 wt% FIrpic (30 nm)/TAZ (50 nm) [8] |
|---|---|
| Colour | Green |
| Max. Luminance | 27,524 cd/m2 |
| Max. Current Efficiency | 71.2 cd/A |
| Device structure | ITO /TAPC/(1wt% DPB:99wt% tri-PXZ-TRZ*):CBP (15:85)/LiF/Al [6] |
|---|---|
| Colour | Red |
| Max EQE | 17.5% |
| Max. Power Efficiency | 28 lm W−1 |
| Device structure | ITO/MO3 (1 nm)/CBP (35 nm)/8 wt% Ir(ppy)2(acac):CBP/TPBi (65 nm)/LiF/Al (100 nm) [7] |
|---|---|
| Colour | Green |
| EQE@100 cd/m2 | 23.4 |
| Current Efficiency@100
cd/m2 |
81 cd/A |
| Powder Efficiency@100
cd/m2 |
78.0 lm W−1 |
| Device structure | ITO/MoOx (2 nm)/m-MTDATA: MoOx (30 nm, 15 wt.%)/m-MTDATA (10 nm)/Ir(ppz)3(10 nm)/CBP:PO-01* (3 nm, 6 wt.%)/Ir(ppz)3 (1 nm)/DBFDPOPhCz*:FIrpic (10 nm,10 wt.%)/Bphen (36 nm)/LiF (1 nm)/Al [9] |
|---|---|
| Colour | White |
| Max. EQE | 12.2% |
| Max. Current Efficiency | 42.4 cd/A |
| Max. Power Efficiency | 47.6 lm W−1 |
| Device structure | ITO/NPB (30 nm)/CBP:8 wt% (t-bt)2Ir(acac)* (15 nm)/BPhen(35 nm)/LiF (1 nm)/CoPc:C60 (4:1) (5 nm)/MoO3(5 nm)/NPB(30 nm)/CBP:8 wt% (t-bt)2Ir(acac)* (15 nm)/BPhen (35 nm)/Mg:Ag (100 nm) [10] |
|---|---|
| Colour | Yellow |
| Max. EQE | 16.78% |
| Max. Luminance | 42,236 cd/m2 |
| Max. Current Efficiency | 50.2 cd/A |
| Max. Power Efficiency | 12.9 lm W−1 |
| Device structure | ITO/NPD* (40 nm)/9%-Ir(piq)3:CBP (20 nm)/BPhen (50 nm)/KF (1 nm)/Al [11] |
|---|---|
| Colour | Red |
| Max. Luminance | 11,000 cd/m2 |
| Max EQE | 10.3% |
| Max. Powder Efficiency | 8.0 lm W−1 |
| Device structure | ITO/0.4 wt% F4TCNQ doped α NPD (30 nm)/ 5 wt% Ir(ppy)3 doped CBP (50 nm)/BPhen (30 nm)/20 wt% TCNQ mixed BPhen (1.5 nm)/Al [12] |
|---|---|
| Colour | Green |
| Luminance@15 V | 1,320 cd/m2 |
| Power Efficiency@14 V | 56.6 lm W−1 |
| Current Efficiency@14 V | 23.17 cd/A |
| Device structure | ITO/F4TCNQ (3 nm)/MeO-Spiro-TPD (27 nm)/CBP + BCzVbi* (50 nm)/BPhen (10 nm)/TCNQ mixed BPhen (1.5 nm)/Al [13] |
|---|---|
| Colour | Red |
| Luminance@ 10 mA/cm2 | 1,790 cd/m2 |
| Power Efficiency@ 10 mA/cm2 | 4.65 lm W−1 |
| Current Efficiency@ 10 mA/cm2 | 18.0 cd/A |
*For chemical structure information, please refer to the cited references.
When fabricating devices, processing and handling materials in a glove box helps maintain their purity and maintain efficiency by avoiding contamination from particulates, moisture, and airborne impurities.
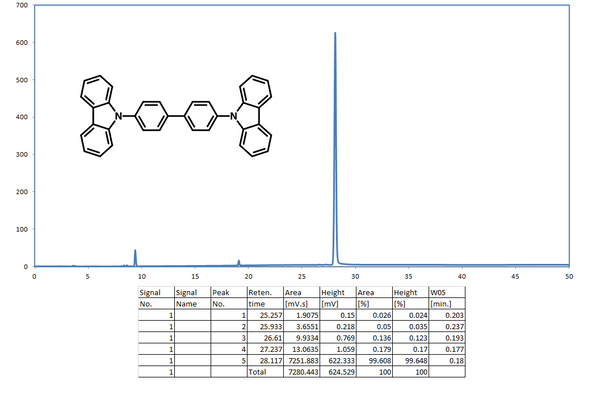
Characterisations
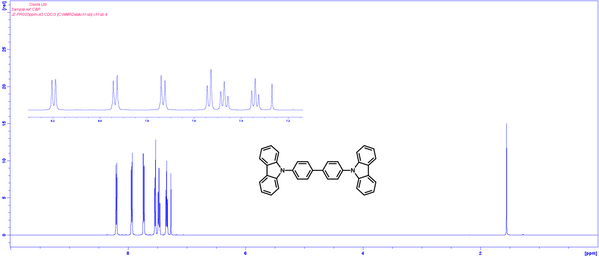
1H NMR of 4,4′-Bis(N-carbazolyl)-1,1′-biphenyl (CBP) in CDCl3.
Pricing
| Grade | Order Code | Quantity | Price |
|---|---|---|---|
| Sublimed (>99.5% purity) | M391 | 1 g | £115 |
| Unsublimed (>98.0% purity) | M392 | 5 g | £180 |
| Sublimed (>99.5% purity) | M391 | 5 g | £480 |
MSDS Documentation
Literature and Reviews
- Transient analysis of organic electrophosphorescence: I. Transient analysis of triplet energy transfer, M. Baldo et al., Phys Rev B, 62: 10958–10966 (2000).
- Management of singlet and triplet excitons for efficient white organic light-emitting devices, Y. Sun, et al, Nature 440, 908-912 (2006), doi:10.1038/nature04645.
- Highly efficient single-layer dendrimer light-emitting diodes with balanced charge transport, T. D. Anthopoulos et al., Appl. Phys. Lett. 82, 4824 (2003).
 CBP MSDS sheet
CBP MSDS sheet