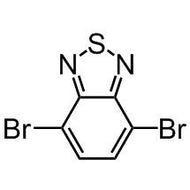
3,6-Dibromothieno[3,2-b]thiophene (TT36)
CAS Number 392662-65-6
Chemistry Building Blocks, Dibromo Monomers, Heterocyclic Building Blocks, Monomers
3,6-Dibromothieno[3,2-b]thiophene (TT36)
Widely used intermediate for further synthesis of semiconducting molecules, oligomers and conjugated polymers
3,6-Dibromothieno[3,2-b]thiophene (TT36), CAS number 392662-65-6, is a derivate of thieno[3,2-b]thiophene with bromine function groups at the 3,6-positions of the fused rings. TT36 is a widely used intermediate to build up more complex structures as semiconducting molecules, oligomers and conjugated polymers for OFETs, sensors, OLEDs and OPVs.

Interestingly, 3,6-dibromothieno[3,2-b]thiophene is prepared by the halogen dance reaction of 2,5-dibromothieno[3,2-b]thiophene with lithium diisopropyl amide (LDA), shown above.
3,6-Dibromothieno[3,2-b]thiophene has a good planar symmetrical structure and functional groups that can be easily modified to optimize the electronic properties. Alkylation or arylation of thieno[3,2-b]thiophene at 3.6-positions can be achieved via Negishi cross-coupling reaction by reacting 3,6-Dibromothieno[3,2-b]thiophene with RMBr (R = alkyl or aryl, M = Zn).
General Information
| CAS Number | 392662-65-6 |
| Chemical Formula | C6H2Br2S2 |
| Full Name | 3,6-Dibromothieno[3,2-b]thiophene |
| Molecular Weight | 298.0 g/mol |
| Synonyms | TT36 |
| Classification / Family | Thienothiophene, semiconductor synthesis intermediates, low band gap polymers, OLED, OFETs, organic photovoltaics |
Chemical Structure
![3,6-Dibromothieno[3,2-b]thiophene (TT36) chemical structure, CAS 392662-65-6](https://cdn.shopify.com/s/files/1/0823/0287/files/3-6-dibromothienothiophene-body-chemical-structure_240x180.png?v=1653663540)
Product Details
| Purity | >98% (1H NMR in CDCl3) |
| Melting Point | 127.0 °C |
| Appearance | Fluffy white/off white powder |
MSDS Documentation
![3,6-Dibromothieno[3,2-b]thiophen MSDS](https://cdn.shopify.com/s/files/1/0823/0287/files/msds-sheets_60x60.jpg) 3,6-Dibromothieno[3,2-b]thiophen MSDS Sheet
3,6-Dibromothieno[3,2-b]thiophen MSDS Sheet
Literature and Reviews
- Fused electron deficient semiconducting polymers for air stable electron transport, A. Onwubiko et al., Nat. Commun. 9, 416 (2018); DOI: 10.1038/s41467-018-02852-6.
- Thieno[3,2-b]thiophene based electrochromic polymers: experimental cum theoretical appraisal of the EDOT position, X. Zhu et al., RSC Adv., 6, 75522 (2016); DOI: 10.1039/c6ra12319h.
- Small Band Gap Polymers Incorporating a Strong Acceptor, Thieno[3,2-b]thiophene-2,5-dione, with P-Channel and Ambipolar Charge Transport Characteristics, I. Osaka et al., J. Mater. Chem. C, 2, 2307-2312 (2014); DOI: 10.1039/C3TC32386B.
- Efficient synthesis of 3,6-dialkoxythieno[3,2-b]thiophenes as precursors of electrogenerated conjugated polymers, N. Hergue et al., Org. Biomol. Chem., 9, 588–595 (2011); DOI: 10.1039/c0ob00585a.
- Dithienylthienothiophenebisimide, a Versatile Electron-Deficient Unit for Semiconducting Polymers, M. Saito et al., Adv. Mater., 28 (32), 6921-6925 (2016); DOI: 10.1002/adma.201601373.
Related Products
We stock a wide range of monomers available to purchase online. Please contact us if you cannot find what you are looking for.
![3,6-Dibromothieno[3,2-b]thiophene (TT36) CAS 392662-65-6](http://www.ossila.com/cdn/shop/products/3-6-dibromothienothiophene-chemical-structure.png?v=1653663075&width=240)
![2-Ethylhexyl 4,6-dibromo-3-fluorothieno[3,4-b]thiophene-2-carboxylate](http://www.ossila.com/cdn/shop/products/ptb7-monomer-b361-ossila-chemical-structure.png?v=1648818400&width=190)
![2,5-bis(trimethylstannyl)-thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/2_5-bis-trimethylstannyl-thieno-3_2-b-thiophene_structure.jpg?v=1504193831&width=190)
![2,6-dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/4Hcyclopentadithiophene.jpg?v=1431610575&width=190)
![2,7-Dibromo-9,9-bis[3,3'-(N,N-dimethylamino)-propyl]fluorene](http://www.ossila.com/cdn/shop/products/dibromo-fluorene-diyl-bisdimethylpropan-amine.jpg?v=1431610994&width=190)
![3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione](http://www.ossila.com/cdn/shop/products/bisbromothiophenyl-bisoctyldodecylpyrrolo-dione.jpg?v=1431611190&width=190)
![4H-Cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/cyclopentadithiophene_12a14774-f96a-4ebc-a0d0-2c5483da9180.jpg?v=1445441165&width=190)
![Benzo[1,2-b:4,5-b']dithiophene-4,8-dione](http://www.ossila.com/cdn/shop/products/benzo-dithiophene-dione.jpg?v=1437904702&width=190)
![Thienothiophene, Thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/thienothiophene.jpg?v=1431611114&width=190)
![Thieno[3,2-b]thiophene-2-carbonitrile](http://www.ossila.com/cdn/shop/products/thienothiophene-2-carbonitrile.jpg?v=1439548051&width=190)
![DTT, Dithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/dtt-chemical-structure.png?v=1653477307&width=190)
![2,3,5,6-Tetrabromothieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/Tetrabromo-thienothiophene-chemical-structure.png?v=1665673773&width=190)
![2,6-Dibromodithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/Dibromodithienothiophene-chemical-structure.png?v=1666702461&width=190)
![2,5-Dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c]pyrrole-1,4-dione](http://www.ossila.com/cdn/shop/products/2_5-Dihydro-3_6-di-2-thienyl-pyrrolo_3_4-c_pyrrole-1_4-dione-chemical-structure-dpp.png?v=1667321819&width=190)
![6,9-bis(5-bromo-4-(2-butyloctyl)thiophen-2-yl)dithieno[3,2-f:2',3'-h]quinoxaline](http://www.ossila.com/cdn/shop/products/bisbromo-butyloctylthiophenyl-dithienoquinoxaline-chemical-structure.png?v=1669202898&width=190)
![Indolo[3,2-b]carbazole](http://www.ossila.com/cdn/shop/products/Indolocarbazole-chemical-structure.png?v=1670495077&width=190)
![10,15-Dihydro-5H-diindolo[3,2-a:3',2'-c]carbazole](http://www.ossila.com/cdn/shop/products/Dihydro-diindolocarbazole-chemical-structure.png?v=1670502109&width=190)
![Indolo[2,3-a]carbazole](http://www.ossila.com/cdn/shop/products/indolo-2-3-a-carbazole-chemical-structure-title.png?v=1678288567&width=190)
![2,2-Bis[4-(4-aminophenoxy)phenyl]propane (BAPP)](http://www.ossila.com/cdn/shop/products/bapp-chemical-structure-title.png?v=1679403349&width=190)
![2,2'-Dimethyl[1,1'-biphenyl]-4,4'-diamine](http://www.ossila.com/cdn/shop/products/2-2-dimethyl1-1-biphenyl-4-4-diamine-chemical-structure-title.png?v=1680597662&width=190)
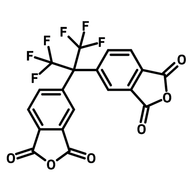
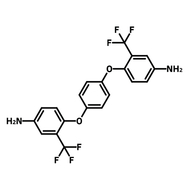
![2,2-Bis[4-(4-aminophenoxy)phenyl]hexafluoropropane (4-BDAF)](http://www.ossila.com/cdn/shop/products/4-bdaf-chemical-structure-title.png?v=1681225583&width=190)
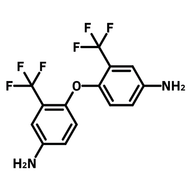
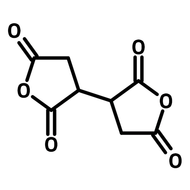
![1-[2-(Trifluoromethyl)phenyl]imidazole](http://www.ossila.com/cdn/shop/files/1-2-trifluoromethylphenylimidazole-chemical-structure-title.png?v=1682593257&width=190)