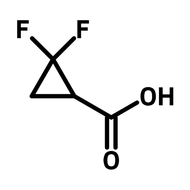
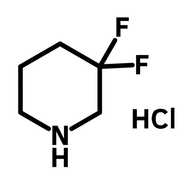
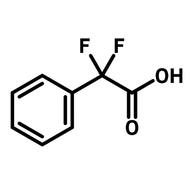
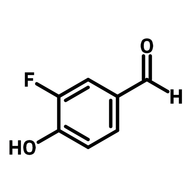
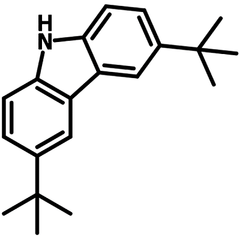
3,6-Di-tert-butylcarbazole
CAS Number 37500-95-1
Chemistry Building Blocks, Heterocyclic Building Blocks, Monomers
An electron donating intermediate for the synthesis of semiconducting molecules
For applications in highly efficient OLEDs and DSSCs.
3,6-Di-tert-butylcarbazole (DtBuCz, CAS number 37500-95-1) is an electron-donating 9H-carbazole derivative with tert-butyl groups at 3,6-positions. The bulky tert-butyl groups can disrupt aggregates that otherwise form dimers or excimers through π–π interactions by enlarging the distance between carbazole rings, leading to loose molecular packing in films.
Carbazoles are typically electrochemically active due to dimerization and polymerization of the corresponding radical cations at their 3,6-positions. The substitutions with tert-butyl groups at 3,6-positions can also suppress such reactions. 3,6-Di-tert-butylcarbazole has also been incorporated in the semiconducting small molecules for OLED applications to gain higher glass-transition temperatures. The introduction of the tert-butyl units can also effectively separate molecules thus reduce the chance of triplet-polaron annihilation to greatly improve the device efficiency and stability.
3,6-Di-tert-butylcarbazole is prepared by Friedel–Crafts alkylation of carbazole with tert-butyl chloride in the presence of aluminium trichloride (AlCl3).
General Information
| CAS Number | 37500-95-1 |
| Chemical Formula | C20H25N |
| Full Name | 3,6-Di-tert-butylcarbazole |
| Molecular Weight | 279.42 g/mol |
| Synonyms | 3,6-Di-tert-butyl-9H-carbazole, Cz-tBu, DtBuCz |
| Classification / Family | Carbazoles, semiconductor synthesis intermediates, Electron donor unit, OLED, OFETs, organic photovoltaics |
Chemical Structure
Product Details
| Purity | >98% (1H NMR) |
| Melting Point | 232 °C |
| Appearance | White to off-white powder/crystals |
MSDS Documentation
 3,6-Di-tert-butylcarbazole MSDS Sheet
3,6-Di-tert-butylcarbazole MSDS Sheet
Literature and Reviews
- Bis(carbazolyl) derivatives of pyrene and tetrahydropyrene: synthesis, structures, optical properties, electrochemistry, and electroluminescence, B. Kaafarani et al., J. Mater. Chem. C, 1, 1638-1650 (2013); DOI: 10.1039/C2TC00474G.
- Steric Effect on Dimer Radical Cation Formation of Poly(3,6-di-tert-butyl-9-vinylcarbazole) and Its Dimeric Model Compounds Studied by Laser Photolysis, Y. Tsujii et al., Polym. J., 22, 319–325 (1990); DOI: 10.1295/polymj.22.319.
- Synthesis and Characterization of 2D-D-π-A-Type Organic Dyes Bearing Bis(3,6-di-tert-butylcarbazol-9-ylphenyl)aniline as Donor Moiety for Dye-Sensitized Solar Cells, T. Khanasa et al., Eur. J. Org. Chem., 13, 2608-2620 (2013); DOI: 10.1002/ejoc.201201479.
- Novel D–A chromophores with condensed 1,2,4-triazine system simultaneously display thermally activated delayed fluorescence and crystallization-induced phosphorescence, A. Maggiore et al., Phys. Chem. Chem. Phys., 24, 17770-17781 (2022); DOI: 10.1039/D2CP00777K.
- Color tuning of dibenzo[a,c]phenazine-2,7-dicarbonitrile-derived thermally activated delayed fluorescence emitters from yellow to deep-red, S. Kothavale et al., J. Mater. Chem. C, 8, 7059-7066 (2020); DOI: 10.1039/d0tc00960a.
- Elucidating the Electronic Structure of a Delayed Fluorescence Emitter via Orbital Interactions, Excitation Energy Components, Charge-Transfer Numbers, and Vibrational Reorganization Energies, Z. Pei et al., J Phys Chem Lett., 12(11): 2712–2720 (2021); DOI: 10.1021/acs.jpclett.1c00094.
Related Products
We stock a wide range of monomers available to purchase online. Please contact us if you cannot find what you are looking for.

![2-Ethylhexyl 4,6-dibromo-3-fluorothieno[3,4-b]thiophene-2-carboxylate](http://www.ossila.com/cdn/shop/products/ptb7-monomer-b361-ossila-chemical-structure.png?v=1648818400&width=190)
![2,5-bis(trimethylstannyl)-thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/2_5-bis-trimethylstannyl-thieno-3_2-b-thiophene_structure.jpg?v=1504193831&width=190)
![2,6-dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/4Hcyclopentadithiophene.jpg?v=1431610575&width=190)
![2,7-Dibromo-9,9-bis[3,3'-(N,N-dimethylamino)-propyl]fluorene](http://www.ossila.com/cdn/shop/products/dibromo-fluorene-diyl-bisdimethylpropan-amine.jpg?v=1431610994&width=190)
![3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione](http://www.ossila.com/cdn/shop/products/bisbromothiophenyl-bisoctyldodecylpyrrolo-dione.jpg?v=1431611190&width=190)
![4H-Cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/cyclopentadithiophene_12a14774-f96a-4ebc-a0d0-2c5483da9180.jpg?v=1445441165&width=190)
![Benzo[1,2-b:4,5-b']dithiophene-4,8-dione](http://www.ossila.com/cdn/shop/products/benzo-dithiophene-dione.jpg?v=1437904702&width=190)
![Thienothiophene, Thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/thienothiophene.jpg?v=1431611114&width=190)
![Thieno[3,2-b]thiophene-2-carbonitrile](http://www.ossila.com/cdn/shop/products/thienothiophene-2-carbonitrile.jpg?v=1439548051&width=190)
![DTT, Dithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/dtt-chemical-structure.png?v=1653477307&width=190)
![3,6-Dibromothieno[3,2-b]thiophene (TT36)](http://www.ossila.com/cdn/shop/products/3-6-dibromothienothiophene-chemical-structure.png?v=1653663075&width=190)
![2,3,5,6-Tetrabromothieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/Tetrabromo-thienothiophene-chemical-structure.png?v=1665673773&width=190)
![2,6-Dibromodithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/Dibromodithienothiophene-chemical-structure.png?v=1666702461&width=190)
![2,5-Dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c]pyrrole-1,4-dione](http://www.ossila.com/cdn/shop/products/2_5-Dihydro-3_6-di-2-thienyl-pyrrolo_3_4-c_pyrrole-1_4-dione-chemical-structure-dpp.png?v=1667321819&width=190)
![6,9-bis(5-bromo-4-(2-butyloctyl)thiophen-2-yl)dithieno[3,2-f:2',3'-h]quinoxaline](http://www.ossila.com/cdn/shop/products/bisbromo-butyloctylthiophenyl-dithienoquinoxaline-chemical-structure.png?v=1669202898&width=190)
![Indolo[3,2-b]carbazole](http://www.ossila.com/cdn/shop/products/Indolocarbazole-chemical-structure.png?v=1670495077&width=190)
![10,15-Dihydro-5H-diindolo[3,2-a:3',2'-c]carbazole](http://www.ossila.com/cdn/shop/products/Dihydro-diindolocarbazole-chemical-structure.png?v=1670502109&width=190)
![Indolo[2,3-a]carbazole](http://www.ossila.com/cdn/shop/products/indolo-2-3-a-carbazole-chemical-structure-title.png?v=1678288567&width=190)
![2,2-Bis[4-(4-aminophenoxy)phenyl]propane (BAPP)](http://www.ossila.com/cdn/shop/products/bapp-chemical-structure-title.png?v=1679403349&width=190)
![2,2'-Dimethyl[1,1'-biphenyl]-4,4'-diamine](http://www.ossila.com/cdn/shop/products/2-2-dimethyl1-1-biphenyl-4-4-diamine-chemical-structure-title.png?v=1680597662&width=190)
![2,2-Bis[4-(4-aminophenoxy)phenyl]hexafluoropropane (4-BDAF)](http://www.ossila.com/cdn/shop/products/4-bdaf-chemical-structure-title.png?v=1681225583&width=190)
![1-[2-(Trifluoromethyl)phenyl]imidazole](http://www.ossila.com/cdn/shop/files/1-2-trifluoromethylphenylimidazole-chemical-structure-title.png?v=1682593257&width=190)