BDTT
CAS Number 1352642-37-5
Chemistry Building Blocks, Heterocyclic Building Blocks, Monomers, Organotin Compounds
BDTT, for applications in OFETs, OLED, PLED and OPV
For the synthesis of small molecules and low band gap semiconducting polymers
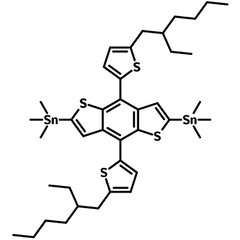
2,6-Bis(trimethytin)-4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b’]dithiophene, or BDTT for short (CAS number 1352642-37-5), has been used for the synthesis of small molecules and low band gap polymer semiconductors such as PTB7, PCE10 and PBDB-T (PCE12) for OFETs, OLED, PLED, OPV applications.
Bearing two ethylhexyl alkyl side chains, with fused thiophenes and thiophenes as electron-rich pendants, 2,6-bis(trimethytin)-4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b’]dithiophene offers good solubility for synthesis of polymer chains.
This monomer has been used in our own lab for the synthesis of PCE10 and PBDB-T (PCE12).
If you are ordering the item with quantity over 5 g please contact our customer care team for a lead time.
Capped with trimethyltin
A monomer for PTB7, PCE10, and PCE12
Benzodithiophene building block
For semiconductors, OFETs, and solar cells
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 1352642-37-5 |
| Chemical Formula | C40H58S4Sn2 |
| Molecular Weight | 904.57 g/mol |
| Synonyms | BDT, BDTT |
| Classification / Family | Thiophene, Fused thiophene, Benzo-dithiophene heterocylic aromatics, Five-membered ring, Semiconductor synthesis intermediates, Low band gap polymers, OFETs, Organic photovoltaics, Polymer solar cells |
Chemical Structure
![1352642-37-5, BDTT, chemical structure 2,6-Bis(trimethytin)-4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b’]dithiophene](https://cdn.shopify.com/s/files/1/0823/0287/files/BDTT-chemical-structure-body_350x263.png?v=1654591133)
Product Details
| Purity | >98% (1H NMR) |
| Melting Point | N/A |
| Appearance | Light yellow crystals |
MSDS Documentation
Literature and Reviews
- Enhanced performance for organic bulk heterojunction solar cells by cooperative assembly of ter(ethylene oxide) pendants, L. Chen et al., Polym. Chem., 5, 4480-4487 (2014); DOI: 10.1039/C4PY00095A.
- Breaking the 10% Efficiency Barrier in Organic Photovoltaics: Morphology and Device Optimization of Well-Known PBDTTT Polymers, Adv. Energy Mater., 6, 1502529 (2016), S. Zhang et al., DOI: 10.1002/aenm.201502529.
- Study of Optical Properties and Molecular Aggregation of Conjugated Low Band Gap Copolymers: PTB7 and PTB7-Th, F. Bencheikh et al., J. Phys. Chem. C 2015, 119, 24643−24648 (2015); DOI: 10.1021/acs.jpcc.5b07803.
Related Products
We stock a wide range of monomers available to purchase online. Please contact us if you cannot find what you are looking for.
 BDTT MSDS Sheet
BDTT MSDS Sheet
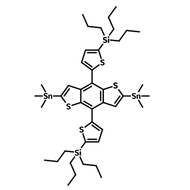
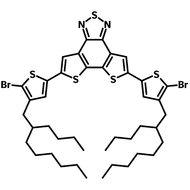
![2-Ethylhexyl 4,6-dibromo-3-fluorothieno[3,4-b]thiophene-2-carboxylate](http://www.ossila.com/cdn/shop/products/ptb7-monomer-b361-ossila-chemical-structure.png?v=1648818400&width=190)
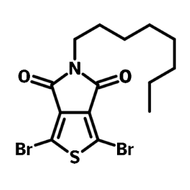
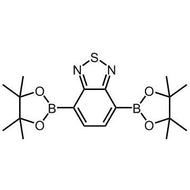
![2,5-bis(trimethylstannyl)-thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/2_5-bis-trimethylstannyl-thieno-3_2-b-thiophene_structure.jpg?v=1504193831&width=190)
![2,6-dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/4Hcyclopentadithiophene.jpg?v=1431610575&width=190)
![2,7-Dibromo-9,9-bis[3,3'-(N,N-dimethylamino)-propyl]fluorene](http://www.ossila.com/cdn/shop/products/dibromo-fluorene-diyl-bisdimethylpropan-amine.jpg?v=1431610994&width=190)
![3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione](http://www.ossila.com/cdn/shop/products/bisbromothiophenyl-bisoctyldodecylpyrrolo-dione.jpg?v=1431611190&width=190)
![4H-Cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/cyclopentadithiophene_12a14774-f96a-4ebc-a0d0-2c5483da9180.jpg?v=1445441165&width=190)
![Benzo[1,2-b:4,5-b']dithiophene-4,8-dione](http://www.ossila.com/cdn/shop/products/benzo-dithiophene-dione.jpg?v=1437904702&width=190)
![Thienothiophene, Thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/thienothiophene.jpg?v=1431611114&width=190)
![Thieno[3,2-b]thiophene-2-carbonitrile](http://www.ossila.com/cdn/shop/products/thienothiophene-2-carbonitrile.jpg?v=1439548051&width=190)
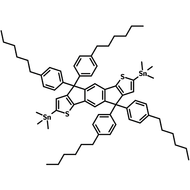
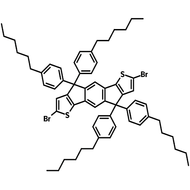
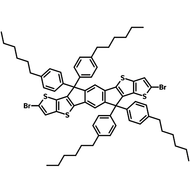
![DTT, Dithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/dtt-chemical-structure.png?v=1653477307&width=190)
![3,6-Dibromothieno[3,2-b]thiophene (TT36)](http://www.ossila.com/cdn/shop/products/3-6-dibromothienothiophene-chemical-structure.png?v=1653663075&width=190)
![2,3,5,6-Tetrabromothieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/Tetrabromo-thienothiophene-chemical-structure.png?v=1665673773&width=190)
![2,6-Dibromodithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/Dibromodithienothiophene-chemical-structure.png?v=1666702461&width=190)
![2,5-Dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c]pyrrole-1,4-dione](http://www.ossila.com/cdn/shop/products/2_5-Dihydro-3_6-di-2-thienyl-pyrrolo_3_4-c_pyrrole-1_4-dione-chemical-structure-dpp.png?v=1667321819&width=190)
![6,9-bis(5-bromo-4-(2-butyloctyl)thiophen-2-yl)dithieno[3,2-f:2',3'-h]quinoxaline](http://www.ossila.com/cdn/shop/products/bisbromo-butyloctylthiophenyl-dithienoquinoxaline-chemical-structure.png?v=1669202898&width=190)
![Indolo[3,2-b]carbazole](http://www.ossila.com/cdn/shop/products/Indolocarbazole-chemical-structure.png?v=1670495077&width=190)
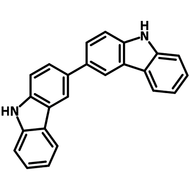
![10,15-Dihydro-5H-diindolo[3,2-a:3',2'-c]carbazole](http://www.ossila.com/cdn/shop/products/Dihydro-diindolocarbazole-chemical-structure.png?v=1670502109&width=190)
![Indolo[2,3-a]carbazole](http://www.ossila.com/cdn/shop/products/indolo-2-3-a-carbazole-chemical-structure-title.png?v=1678288567&width=190)
![2,2-Bis[4-(4-aminophenoxy)phenyl]propane (BAPP)](http://www.ossila.com/cdn/shop/products/bapp-chemical-structure-title.png?v=1679403349&width=190)
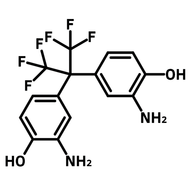
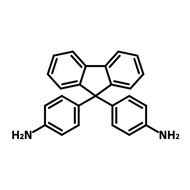
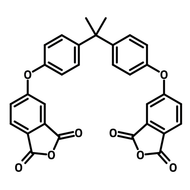
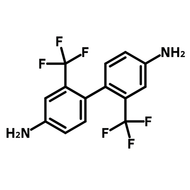
![2,2'-Dimethyl[1,1'-biphenyl]-4,4'-diamine](http://www.ossila.com/cdn/shop/products/2-2-dimethyl1-1-biphenyl-4-4-diamine-chemical-structure-title.png?v=1680597662&width=190)
![2,2-Bis[4-(4-aminophenoxy)phenyl]hexafluoropropane (4-BDAF)](http://www.ossila.com/cdn/shop/products/4-bdaf-chemical-structure-title.png?v=1681225583&width=190)
![1-[2-(Trifluoromethyl)phenyl]imidazole](http://www.ossila.com/cdn/shop/files/1-2-trifluoromethylphenylimidazole-chemical-structure-title.png?v=1682593257&width=190)