2,5-bis(trimethylstannyl)-thieno[3,2-b]thiophene
CAS Number 469912-82-1
Chemistry Building Blocks, Heterocyclic Building Blocks, Monomers, Organotin Compounds
High-purity monomer used in organic electronic devices
High-quality and expert support available online
2,5-bis(trimethylstannyl)thieno[3,2-b]thiophene, CAS number 469912-82-1, can be prepared by using thieno[3,2-b]thiophene as starting material, further reaction with dibromo compounds (Stille Coupling) to form oligomers or polymers which can be used for organic electronic devices.
Thienothiophene is fused with extend conjugation over two thiophene rings. Thienothiophene is proven to be a good candidate for the materials that are used for Organic Field Effect Transistors, Sensors and OPV devices. Thienothiophene provide further tuning of energy gap of polymer materials with higher electron density over the conjugated ring systems.
![dppt-co-tt synthesis with 2,5-bis(trimethylstannyl)thieno[3,2-b]thiophene and 3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione](http://cdn.shopify.com/s/files/1/0823/0287/files/DPPT-co-TT-synthesis_636x142.jpg?164803159279833831)
Note: This product has been used in our own lab by Ossila chemists for the synthesis of DPP-DTT (high mobility p-type polymer), one of our most popular products for optoelectronic devices.
Capped with trimethyltin
For facil coupling reactions
Thienothiophene building block
For semiconductors, OFETs, and solar cells
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 469912-82-1 |
| Chemical Formula | C12H20S2Sn2 |
| Molecular Weight | 465.84 g/mol |
| Synonyms |
|
| Classification / Family | Thiophene, Thienothiophene, Fused thiophene, Heterocyclic five-membered ring, Organic materials, Semiconductor synthesis, Low band gap polymers, OFETs, Organic photovoltaics, Polymer solar cells |
Chemical Structure
![Chemical structure of 2,5-bis(trimethylstannyl)thieno[3,2-b]thiophene, CAS 469912-82-1](https://cdn.shopify.com/s/files/1/0823/0287/products/trimethylstannyl-thienothiophene_240x126.jpg?v=1504193852)
Product Details
| Purity | >98% (NMR) |
| Melting Point | 128 °C - 131 °C |
| Appearance | White crystals |
Charaterisation
MSDS Documentation
![2,5-bis(trimethylstannyl)-thieno[3,2-b]thiophene MSDS](https://cdn.shopify.com/s/files/1/0823/0287/files/msds-sheets_60x60.jpg) 2,5-bis(trimethylstannyl)-thieno[3,2-b]thiophene MSDS sheet
2,5-bis(trimethylstannyl)-thieno[3,2-b]thiophene MSDS sheet
Literature and Reviews
- A High Mobility P-Type DPP-Thieno[3,2-b]thiophene Copolymer for Organic Thin-Film Transistors, Y. Li et al., Adv. Mater., 22, 4862-4866 (2010)
- Thieno[3,2-b]thiophene-Bridged D−π–A Polymer Semiconductor Based on Benzo[1,2-b:4,5-b′]dithiophene and Benzoxadiazole, X. Wang et al., Macromolecules, 46 (12), 4805-4812 (2013)
- Liquid-crystalline semiconducting polymers with high charge-carrier mobility, I. Mcculloch et al., Nat. Mater., 5, 328 (2006).
- Thieno[3,2-b]thiophene-Substituted Benzo[1,2-b:4,5-b′]dithiophene as a Promising Building Block for Low Bandgap Semiconducting Polymers for High-Performance Single and Tandem Organic Photovoltaic Cells, J-H. Kim et al., Chem. Mater., 26 (2), 1234–1242 (2014).
Related Products
We stock a wide range of monomers available to purchase online. Please contact us if you cannot find what you are looking for.
![2,5-bis(trimethylstannyl)-thieno[3,2-b]thiophene CAS 469912-82-1](http://www.ossila.com/cdn/shop/products/2_5-bis-trimethylstannyl-thieno-3_2-b-thiophene_structure.jpg?v=1504193831&width=240)
![2-Ethylhexyl 4,6-dibromo-3-fluorothieno[3,4-b]thiophene-2-carboxylate](http://www.ossila.com/cdn/shop/products/ptb7-monomer-b361-ossila-chemical-structure.png?v=1648818400&width=190)
![2,6-dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/4Hcyclopentadithiophene.jpg?v=1431610575&width=190)
![2,7-Dibromo-9,9-bis[3,3'-(N,N-dimethylamino)-propyl]fluorene](http://www.ossila.com/cdn/shop/products/dibromo-fluorene-diyl-bisdimethylpropan-amine.jpg?v=1431610994&width=190)
![3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione](http://www.ossila.com/cdn/shop/products/bisbromothiophenyl-bisoctyldodecylpyrrolo-dione.jpg?v=1431611190&width=190)
![4H-Cyclopenta[1,2-b:5,4-b']dithiophene](http://www.ossila.com/cdn/shop/products/cyclopentadithiophene_12a14774-f96a-4ebc-a0d0-2c5483da9180.jpg?v=1445441165&width=190)
![Benzo[1,2-b:4,5-b']dithiophene-4,8-dione](http://www.ossila.com/cdn/shop/products/benzo-dithiophene-dione.jpg?v=1437904702&width=190)
![Thienothiophene, Thieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/thienothiophene.jpg?v=1431611114&width=190)
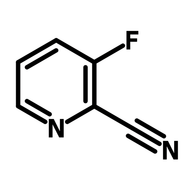
![Thieno[3,2-b]thiophene-2-carbonitrile](http://www.ossila.com/cdn/shop/products/thienothiophene-2-carbonitrile.jpg?v=1439548051&width=190)
![DTT, Dithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/dtt-chemical-structure.png?v=1653477307&width=190)
![3,6-Dibromothieno[3,2-b]thiophene (TT36)](http://www.ossila.com/cdn/shop/products/3-6-dibromothienothiophene-chemical-structure.png?v=1653663075&width=190)
![2,3,5,6-Tetrabromothieno[3,2-b]thiophene](http://www.ossila.com/cdn/shop/products/Tetrabromo-thienothiophene-chemical-structure.png?v=1665673773&width=190)
![2,6-Dibromodithieno[3,2-b:2′,3′-d]thiophene](http://www.ossila.com/cdn/shop/products/Dibromodithienothiophene-chemical-structure.png?v=1666702461&width=190)
![2,5-Dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c]pyrrole-1,4-dione](http://www.ossila.com/cdn/shop/products/2_5-Dihydro-3_6-di-2-thienyl-pyrrolo_3_4-c_pyrrole-1_4-dione-chemical-structure-dpp.png?v=1667321819&width=190)
![6,9-bis(5-bromo-4-(2-butyloctyl)thiophen-2-yl)dithieno[3,2-f:2',3'-h]quinoxaline](http://www.ossila.com/cdn/shop/products/bisbromo-butyloctylthiophenyl-dithienoquinoxaline-chemical-structure.png?v=1669202898&width=190)
![Indolo[3,2-b]carbazole](http://www.ossila.com/cdn/shop/products/Indolocarbazole-chemical-structure.png?v=1670495077&width=190)
![10,15-Dihydro-5H-diindolo[3,2-a:3',2'-c]carbazole](http://www.ossila.com/cdn/shop/products/Dihydro-diindolocarbazole-chemical-structure.png?v=1670502109&width=190)
![Indolo[2,3-a]carbazole](http://www.ossila.com/cdn/shop/products/indolo-2-3-a-carbazole-chemical-structure-title.png?v=1678288567&width=190)
![2,2-Bis[4-(4-aminophenoxy)phenyl]propane (BAPP)](http://www.ossila.com/cdn/shop/products/bapp-chemical-structure-title.png?v=1679403349&width=190)
![2,2'-Dimethyl[1,1'-biphenyl]-4,4'-diamine](http://www.ossila.com/cdn/shop/products/2-2-dimethyl1-1-biphenyl-4-4-diamine-chemical-structure-title.png?v=1680597662&width=190)
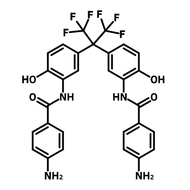
![2,2-Bis[4-(4-aminophenoxy)phenyl]hexafluoropropane (4-BDAF)](http://www.ossila.com/cdn/shop/products/4-bdaf-chemical-structure-title.png?v=1681225583&width=190)
![1-[2-(Trifluoromethyl)phenyl]imidazole](http://www.ossila.com/cdn/shop/files/1-2-trifluoromethylphenylimidazole-chemical-structure-title.png?v=1682593257&width=190)