Liquid Crystals
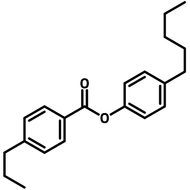
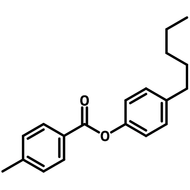
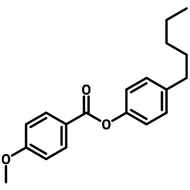
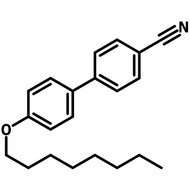
Liquid crystals exist in a state between conventional liquids and crystalline solids. A liquid can flow freely and is isotropic which means its properties are uniform in all directions. A crystalline solid is anisotropic which means its molecules are orientally packed and may not flow. A liquid crystal can flow like a liquid, but its molecules are oriented in a crystal-like way.
The dual nature of liquid crystals allows them to flow like liquids while exhibiting directional optical, electrical, and thermal properties like crystals.
Browse Liquid Crystals
Related categories: photonic and optical materials, liquid crystals
More on Liquid Crystals
Liquid crystals can also be categorised as either thermotropic or lyotropic. Lyotropic liquid crystals have a phase transition as a function of both temperature and concentration of the liquid-crystal molecules in a solvent. An everyday example of a lyotropic liquid crystal is soap.
Thermotropic liquid crystals have a phase transition into the liquid-crystalline state as temperature is raised from their solid state or as temperature is lowered from their liquid state. Among the thermotropic liquid crystals there are two different classes of substances, those are either enantiotropic or monotropic. Enantiotropic liquid crystals are able to enter the liquid crystal state both by cooling a liquid and heating a solid. Monotropic liquid crystals, on the other hand, can enter their liquid crystal phases by either cooling a liquid or heating a solid, but not both.