A PhD Student Condenses: ITIC & Derivatives as OPV Acceptors
Condensed Summary
Title: Effect of Non-Fullerene Acceptors’ Side Chains on the Morphology and Photovoltaic Performance of Organic Solar Cells
Citation: C. Zhang, S. Feng, Y. Liu, R. Hou, Z. Zhang, X. Xu, Y. Wu and Z. Bo, ACS Appl. Mater. Interfaces, 2017, 9, 33906-33912
DOI: 10.1021/acsami.7b09915
Learning point: Side chain modification of electron acceptors can be used to optimise bulk heterojunction morphology, and performance.
ITIC and Its Derivatives

Of the significant efforts in research devoted to NFAs, the proposal of the fused-ring system 3,9-bis(2-methylene-(3-(1,1-dicyanomethylene)-indanone))-5,5,11,11-tetrakris(4-hexylphenyl)-dithieno[2,3-d:2’,3’-d’]-s-indaceno[1,2-b:5,6-b’]dithiophene, or ‘ITIC’, in 2015 has generated the most success. In the initial paper proposing ITIC, a power conversion efficiency (PCE) of 6.80% was achieved in combination with a PTB7-TH donor.5 Efficiencies above 12% have been measured with various ITIC derivatives, such as ITIC-M,6 ITIC-2F7 and ITIC-Th.8
Why is ITIC a Good Acceptor?
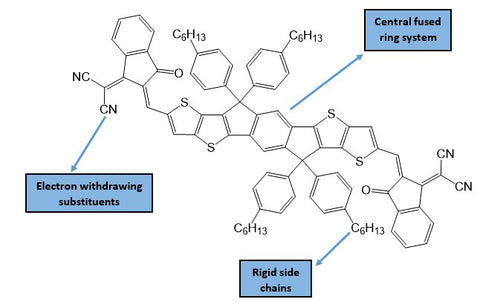
In the recent paper by Zhang et al., the authors noted that ITIC performs well because the extended conjugation in its core of fused aromatic rings allows better charge transport between molecules.9
Emma's comments: Acceptors like ITIC - with a fused aromatic system - are known as fused-ring electron acceptors (FREAs). This also includes earlier NFAs, such as those based on rylene diimides.10
Typically, fused-ring systems will be modified with electron-withdrawing substituents (such as halogens) because these moieties lower the molecular energy levels of the acceptor, and allow energy-level matching with the electron donor.11 Rigid side-chains are also favourable, as these will prevent significant aggregation of the molecules, thus giving better bulk heterojunction (BHJ) morphology.
Emma's comments: Reduced aggregation is also a benefit of 3-dimensional acceptors like PCBM.2 This is in contrast to many planar acceptors that can form extended crystalline domains, which are unfavourable for BHJ morphology.
How Can We Make ITIC Better?
In the article, the authors modified ITIC with three different alkylthio-based side-chains, which can be seen in the figure below. This resulted in a yield of three ITIC analogues: ITIC-SC6, ITIC-SC8 and ITIC-SC2C6.

Emma's comments: Most common derivatives of ITIC (such as ITIC-Th) modify the core structure itself, but side-chain modification can also yield interesting results. The chemical nature of the side-chain, its position, bulkiness and length can all impact features such as solubility, intermolecular interactions, electronic properties and morphology.
The impact on solar cell performance from each side-chain was assessed, and the results of this can be seen in the table below. The difference in results is proposed to be due to different solubility levels of the side-chains in the dichlorobenzene solvent. ITIC-SC2C6 showed the highest solubility in the solvent, and thus gave the most favourable BHJ morphology. Transmission electron microscope (TEM) images showed that with this acceptor, the donor was more likely to aggregate into nanofibril structures - giving an extended interpenetrating network, ideal for a BHJ active layer.

Final Thoughts
The main conclusion that can be drawn from this paper is that small changes in solubility of a molecule (due to alterations of side-chains) can have a significant impact on morphology. In order to gain optimum morphology for a BHJ cell, several side-chain combinations may need to be trialled, and the ideal acceptor could significantly increase the PCE of a system. Further investigation of the impact of side-chains on fused ring systems can be found in several other works.12-14
Materials Mentioned in This Paper
If you are interested in conducting a similar experiment, some of these materials are available from Ossila.References
-
Effect of Non-fullerene Acceptors' Side Chains on the Morphology and Photovoltaic Performance of Organic Solar Cells, C. Zhang et al., ACS Appl. Mater. Interfaces, 9, 33906-33912 (2017); DOI: 10.1021/acsami.7b09915
-
Efficient Organic Solar Cells with Non-Fullerene Acceptors, S. Li et al., Small, 13, 2017; DOI: 10.1002/adma.201404317
-
Non-fullerene Electron Acceptors for Use in Organic Solar Cells, C. B. Nielsen et al., Acc. Chem. Res., 48, 2803-2812 (2015); DOI: 10.1021/acs.accounts.5b00199
-
Recent Progress in Non-fullerene Small Molecule Acceptors in Organic Solar Cells (OSCs), W. Chen et al., J. Mater. Chem. C, 5, 1275-1302 (2017); DOI: 10.1039/C6TC05066B.
-
An Electron Acceptor Challenging Fullerenes for Efficient Polymer Solar Cells, Y. Lin et al., Adv. Mater., 27, 1170-1174 (2015); DOI: 10.1002/adma.201404317.
-
Energy-Level Modulation of Small-Molecule Electron Acceptors to Achieve Over 12% Efficiency in Polymer Solar Cells, S. Li et al., Adv. Mater., 28, 9423-9429 (2016); DOI: 10.1002/adma.201602776
-
Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells, W. Zhao et al. J. Am. Chem. Soc., 139, 7148-7151( 2017); DOI: 10.1021/jacs.7b02677.
- High-Performance Electron Acceptor with Thienyl Side Chains for Organic Photovoltaics, Y. Lin et al., J. Am. Chem. Soc., 138, 4955-4961 (2016); DOI: 10.1021/jacs.6b02004.
-
Structure Evolution of Oligomer Fused-Ring Electron Acceptors toward High Efficiency of As-Cast Polymer Solar Cells, Y. Lin et al., Adv. Energ. Mater., 6, (2016); DOI: 10.1002/aenm.201600854.
-
Non-fullerene Small Molecule Acceptors Based on Perylene Diimides, Z. Liu et al., J. Mater. Chem. A, 4, 17604-17622 (2016); DOI: 10.1039/C6TA06978A.
- Designing Efficient Non-Fullerene Acceptors by Tailoring Extended Fused-Rings with Electron-Deficient Groups, Y. Lin et al., Adv. Energ. Mater., 5 (2016); DOI: 10.1002/aenm.201501063.
-
Side-Chain Isomerization on an n-type Organic Semiconductor ITIC Acceptor Makes 11.77% High Efficiency Polymer Solar Cells, Y. Yang et al., J. Am. Chem. Soc., 138, 15011-15018 (2016).; DOI: 10.1021/jacs.6b09110.
-
Enhancing performance of non-fullerene organic solar cells via side chain engineering of fused-ring electron acceptors, C. Yan et al., Dyes Pigm., 139, 627-634 (2017); DOI: 10.1016/j.dyepig.2016.12.065.
-
Simultaneous enhancement of the molecular planarity and the solubility of non-fullerene acceptors: effect of aliphatic side-chain substitution on the photovoltaic performance, Z. Zhang et al., J. Mater. Chem. A, 5, 7776-778 (2017); DOI: 10.1039/C7TA02141K.




